
Abstract
Aims: We conducted this study to evaluate the efficacy of drug-coated balloon therapy for in-stent restenosis after coronary bifurcation stenting.
Methods and results: Patients who underwent angioplasty with at least one paclitaxel-coated balloon for in-stent restenosis after bifurcation intervention using a two-stent approach were included. Two types of paclitaxel-coated balloon were used, with either an iopromide (iopromide-PCB) or a butyryl tri-n-hexyl citrate (BTHC-PCB) excipient. Angiographic surveillance was planned at six to eight months. Quantitative coronary angiography analysis was carried out with dedicated bifurcation analysis software. Clinical follow-up was performed to one year. In total, 177 patients were included in this study. Information on the type of stent technique used at the time of the index intervention was available for 145 (81.9%) patients: the culotte technique was used in 123 (69.5%) and T-stenting in 22 (12.4%) patients. Iopromide-PCB and BTHC-PCB were used in 124 (70%) and 53 (30%) patients, respectively. Of 125 patients who underwent angiographic follow-up, 30 cases (24%) of binary restenosis were observed. At one year, the composite endpoint of death, myocardial infarction or target lesion revascularisation was observed in 35 patients (24%). There was no significant difference in the incidence of angiographic and clinical outcomes between iopromide-PCB versus BTHC-PCB.
Conclusions: In the setting of in-stent restenosis after coronary bifurcation stenting, drug-coated balloons demonstrated good clinical efficacy without the requirement for further stent implantation. There were similar outcomes between iopromide-PCB and BTHC-PCB.
Abbreviations
BTHC: butyryl tri-n-hexyl citrate
DCB: drug-coated balloon
DES: drug-eluting stent
DS: diameter stenosis
ISR: in-stent restenosis
KBA: kissing balloon angioplasty
LLL: late lumen loss
MACE: major adverse cardiac events
PCB: paclitaxel-coated balloon
Introduction
The incidence of in-stent restenosis (ISR) after percutaneous intervention at coronary bifurcation sites remains considerable and the optimal management is not well defined1. In general, two approaches seem most promising: repeat stenting with drug-eluting stents (DES) or angioplasty with drug-coated balloons (DCB)2. However, some concern exists regarding the long-term fate of patients with ISR treated with repeat stenting3. This may be related to the adverse effects of multiple layers of stent and polymer in the vessel wall4. Indeed, this issue is of magnified relevance in cases of ISR after bifurcation stenting when multiple stent layers already exist.
In this respect, DCB angioplasty might be the preferred treatment strategy for ISR after bifurcation stenting5. However, although midterm to long-term efficacy and safety of DCB have been shown to be similar to those achieved with DES across the spectrum of in-stent restenotic lesions in a study powered for angiographic endpoints3, there is a paucity of data concerning the outcomes of patients with restenosis at the site of a bifurcation lesion6,7. Moreover, prior studies have not included detailed angiographic analysis during surveillance after DCB angioplasty for bifurcation ISR.
Against this background, we sought to evaluate the precise angiographic efficacy of DCB for ISR at bifurcation sites and to determine the comparative efficacy of two DCB devices in this setting.
Methods
STUDY DESIGN AND PATIENT SELECTION
This was a retrospective study including consecutive patients who underwent coronary angioplasty with at least one paclitaxel-coated balloon, coated with either iopromide excipient (iopromide-PCB) (SeQuent Please®; B. Braun Melsungen AG, Melsungen, Germany) or butyryl tri-n-hexyl citrate excipient (BTHC-PCB) (Pantera Lux; Biotronik, Bülach, Switzerland) for ISR after coronary bifurcation stenting with DES using a two-stent approach between July 2009 and September 2014 at two centres in Munich, Germany - Deutsches Herzzentrum München and 1. Medizinische Klinik, Klinikum Rechts der Isar. There were no exclusion criteria.
STUDY DEVICES AND PROCEDURES
Both iopromide-PCB and BTHC-PCB are coated with 3 µg paclitaxel per mm2, using iopromide and butyryl tri-n-hexyl citrate as their respective excipients8,9. The interventional strategy (including the selection of DCB type and number, predilatation with an uncoated balloon, use of a cutting balloon, stenting after DCB and kissing balloon angioplasty [KBA]) was performed according to protocol if patients were enrolled in a randomised trial or at the operator’s discretion if not. All patients were pre-treated with an oral loading dose of aspirin and an ADP receptor antagonist. All patients received intravenous heparin during the intervention with adjunctive antithrombotic therapy administered at the discretion of the operator.
FOLLOW-UP
In line with standard institutional practice, patients were recommended to receive aspirin and clopidogrel for at least six months following the intervention. Other cardiac drugs were prescribed according to the judgement of the patient’s physician. Follow-up angiography was scheduled at six to eight months after the index procedure. Clinical follow-up was performed by either telephone contact or office visit at 30 days and one year after the index procedure.
QUANTITATIVE CORONARY ANGIOGRAPHIC MEASUREMENTS
Quantitative coronary angiography (QCA) analysis of coronary angiograms carried out pre and post procedure and at follow-up was carried out using the bifurcation analysis application in the QAngioXA version 7.1 software system (Medis medical imaging systems bv, Leiden, the Netherlands). In the evaluation of bifurcation segments, measurements were made of the three components, as shown in Figure 1: the main vessel proximal (with boundaries defined by the proximal delimiter and 5 mm proximal to same); the main vessel distal (with boundaries defined by the carina point and 5 mm distal to same); and the side branch (with boundaries defined by the carina point and 5 mm distal to same). Reference vessel diameter, minimal lumen diameter and percent diameter stenosis (%DS) were measured in the respective three components. Late lumen loss (LLL) was defined as minimal lumen diameter post intervention minus that at angiographic follow-up. In addition to data from the three individual components, %DS and LLL of the most severely stenotic component in each bifurcation were recorded as maximal %DS and maximal LLL.

Figure 1. Schema for quantitative coronary angiographic analysis using software dedicated for bifurcation analysis. A) Main vessel proximal. B) Main vessel distal. C) Side branch. Arrowhead: carina point.
STUDY OUTCOMES AND DEFINITIONS
Bifurcation lesions were classified according to the Medina classification10. True bifurcation lesions were defined as the Medina classification of 1, 1, 1; 1, 0, 1; or 0, 1, 1. The primary endpoint of interest was binary restenosis at the bifurcation site, defined as a %DS of 50% or more in any one of the three components of the bifurcation at angiographic follow-up. The secondary endpoints of interest included LLL and major adverse cardiac events (MACE), defined as the composite of all-cause death, myocardial infarction and target lesion revascularisation within one year after the index procedure. Target lesion revascularisation was defined as any repeat percutaneous coronary intervention or bypass surgery to treat the target bifurcation lesion.
STATISTICAL ANALYSIS
Baseline clinical characteristics and procedural information were retrospectively collected at the two participating centres. Characteristics and outcomes between patients treated with iopromide-PCB versus BTHC-PCB as well as patients treated with KBA versus those treated without KBA were compared. Continuous variables are presented as mean±standard deviation and compared using a Kruskal-Wallis rank-sum test. Categorical or binary variables are presented as number (percentage, 95% confidence interval [as applicable]) and compared using the chi-squared test or Fisher’s exact test. Event-free survival was assessed using the Kaplan-Meier method, and hazard ratios, 95% confidence intervals and p-values were calculated from univariate Cox proportional hazards models. P-values less than 0.05 were considered statistically significant. Statistical software S-PLUS, version 4.5 (Insightful Corp., Seattle, WA, USA) was used for analysis.
Results
BASELINE CHARACTERISTICS
One hundred and seventy-seven patients were included in this study. Baseline patient characteristics are shown in Table 1. In total, the mean age of the patients was 68 years, 86% were male, 43% had diabetes mellitus and 29% presented with acute coronary syndrome at the time of the index procedure. Information on the type of stent technique used at the time of the index intervention was available for 145 (81.9%) patients: the culotte technique was used in 123 (69.5%) and T-stenting in 22 (12.4%) patients.

ANGIOGRAPHIC AND PROCEDURAL CHARACTERISTICS
Baseline angiographic and procedural characteristics are shown in Table 2. Forty-six percent of lesions involved the left main coronary artery bifurcation and the incidence of true bifurcation lesions was 53%. The distribution of the site of maximal %DS in each included lesion is shown in Figure 2. Iopromide-PCB and BTHC-PCB were used in 124 (70%) and 53 (30%) patients, respectively. KBA was performed in 109 patients (62%). The frequency of cutting balloon use during the intervention was significantly different between the iopromide-PCB versus BTHC-PCB groups (10% versus 26%, respectively, p<0.01) and between the KBA versus no KBA groups (10% versus 22%, respectively, p=0.03).
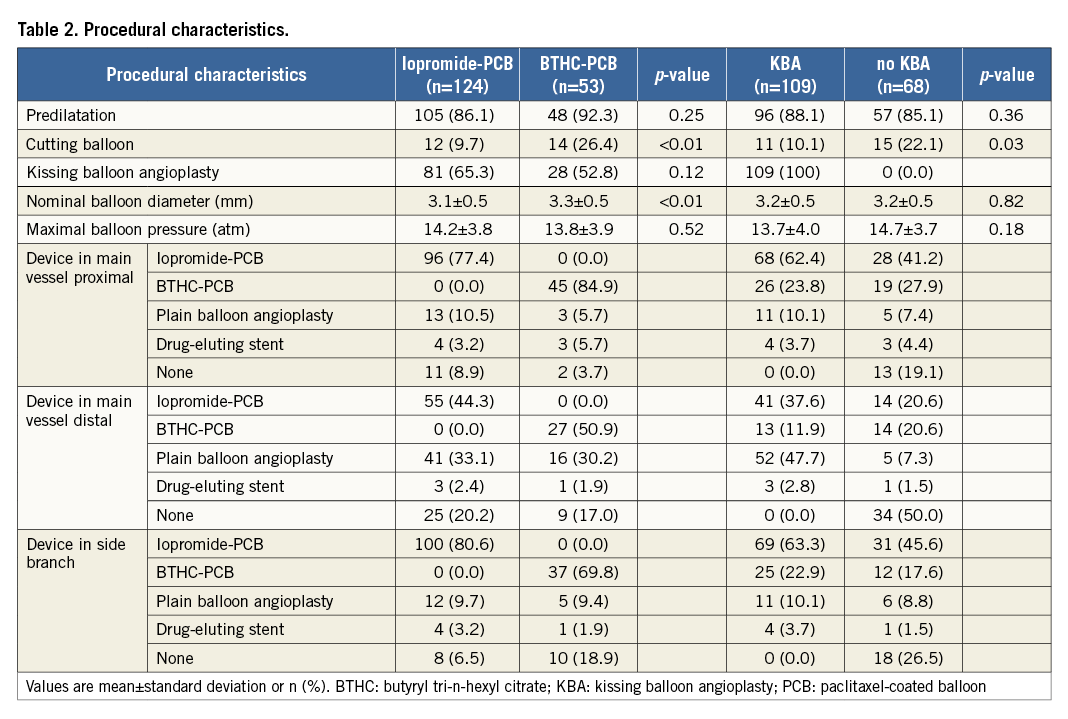
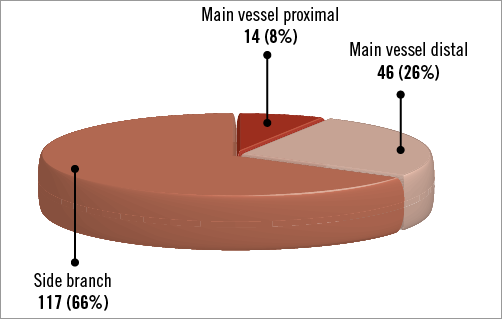
Figure 2. Distribution of the site of maximal percent diameter stenosis pre procedure.
ANGIOGRAPHIC OUTCOMES
The angiographic results are shown in Table 3. One hundred and twenty-five patients underwent angiographic follow-up at a median time of 199 days after the index procedure. Among these patients, 30 cases (24%, 95% confidence interval [CI]: 17%-32%) of binary restenosis were observed, most commonly in the side branch (70%, 95% CI: 54%-86%) followed by the main vessel distal (27%, 95% CI: 16%-43%) and the main vessel proximal (3%, 95% CI: 0%-10%). There was no difference in the incidence of binary restenosis between patients treated with iopromide-PCB (25%, 95% CI: 16%-34%) versus BTHC-PCB (21%, 95% CI: 8%-34%) (p=0.61), or between those treated with KBA (25%, 95% CI: 16%-35%) versus without KBA (22%, 95% CI: 10%-34%) (p=0.73) (Figure 3A). Overall, the maximal LLL was 0.45 mm and %DS was 37.2%. There was no significant difference in the maximal LLL or the maximal %DS at follow-up between the iopromide-PCB versus BTHC-PCB groups, or between with KBA versus no KBA groups (Figure 3B, Figure 3C).

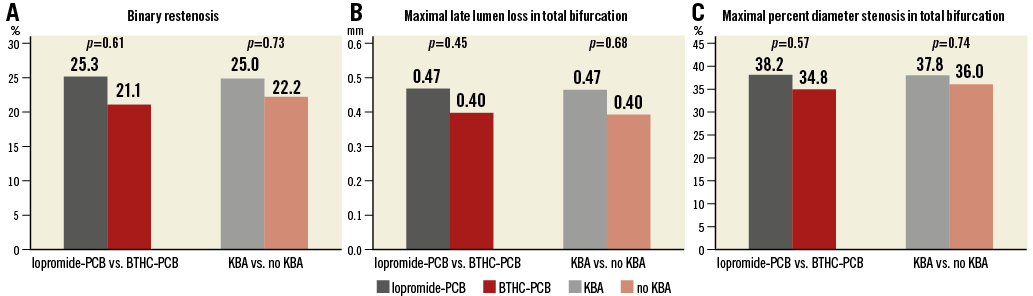
Figure 3. Angiographic outcomes at follow-up. A) Binary restenosis. B) Maximal late lumen loss in total bifurcation. C) Maximal percent diameter stenosis in total bifurcation. BTHC: butyryl tri-n-hexyl citrate; KBA: kissing balloon angioplasty; PCB: paclitaxel-coated balloon
CLINICAL OUTCOMES
Completed follow-up at one year was available in 135 patients (76%); in patients who did not have complete one-year follow-up, the median duration of follow-up was 160 (18-231) days. At one year after the index procedure, MACE was observed in 35 patients (24%, 95% CI: 16%-30%). The incidences of each component of MACE were as follows: all-cause death, three (2%, 95% CI: 0%-4%); myocardial infarction, zero (0%); and target lesion revascularisation, 32 (22%, 95% CI: 15%-28%). There were no significant differences in estimated rates of MACE at one year between the iopromide-PCB group (26%, 95% CI: 17%-34%) versus the BTHC-PCB group (17%, 95% CI: 5%-27%) (hazard ratio=1.66, 95% CI: 0.73-3.80; p=0.23) (Figure 4A), or between the KBA group (22%, 95% CI: 13%-30%) versus the no KBA group (26%, 95% CI: 14%-37%) (hazard ratio=0.83, 95% CI: 0.42-1.62; p=0.58) (Figure 4B). No cases of stent thrombosis were observed.

Figure 4. Time-to-event curve for incidence of the composite outcome of death, myocardial infarction or target lesion revascularisation (MACE). A) Iopromide-PCB versus BTHC-PCB. B) KBA versus no KBA. Hazard ratios and p-values are derived from Cox proportional hazard models. BTHC: butyryl tri-n-hexyl citrate; CI: confidence interval; HR: hazard ratio; KBA: kissing balloon angioplasty; MACE: major adverse cardiac events; PCB: paclitaxel-coated balloon
Discussion
The main findings of our study were as follows. First, in patients with ISR after prior bifurcation stenting, who were treated with DCB angioplasty, the incidence of recurrent binary restenosis and the rate of MACE within one year of intervention were satisfactory. Second, angiographic and clinical outcomes were similar for lesions treated with iopromide-PCB versus BTHC-PCB, and for those treated with final KBA versus without.
ISR following drug-eluting stenting is generally challenging to treat. Rates of subsequent events seem to be higher in comparison with rates after treatment of bare metal stent ISR and the optimal management strategy is somewhat unclear11,12. In a recent network meta-analysis, the two strategies which showed the best results were repeat stenting with second-generation DES and angioplasty with DCB2. Although repeat stenting showed somewhat higher clinical efficacy, angioplasty with DCB avoids the need for additional stent layers. Moreover, repeat stenting might be associated with an increase in late adverse events3. Indeed, this issue is of particular relevance in the setting of repeat intervention for restenosis in bifurcation lesions already treated with a two-stent approach.
In the current study, DCB showed high efficacy in terms of both angiographic and clinical outcomes after treatment for ISR after bifurcation stenting: the incidence of binary restenosis at surveillance angiography at a median follow-up of 199 days was 24% and the rate of MACE at one year was 24%. These observations are consistent with findings of prior studies of DCB treatment for ISR at bifurcation sites6,7, and comparable to the results of recent reports regarding the efficacy of DCB in coronary ISR in any location which show MACE rates of 4.0-23.5%13-17. Moreover, DCB angioplasty in this setting also demonstrated excellent midterm safety in our study, with no occurrence of stent thrombosis. This is noteworthy, as intervention at the site of a coronary bifurcation is known to be an independent risk factor for stent thrombosis18. On the basis of our data, we suggest that angioplasty with DCB might be an attractive option for the management of ISR after coronary bifurcation stenting.
In our study, there was no significant relationship between the type of DCB used for treatment and angiographic and clinical outcomes. Indeed, randomised comparison studies between different DCB types for both bifurcation and non-bifurcation lesions are lacking, and the rationale for conducting our analysis was the preclinical evidence of differential efficacy between balloons19. Of course, the findings of our study in this respect must be interpreted with caution due to the non-randomised nature of treatment allocation. Indeed, there were important differences in baseline and procedural characteristics between patients treated with different balloon types, e.g., the proportion of left main coronary artery bifurcation lesions was lower (39%) in the iopromide-PCB versus the BTHC-PCB group (62%), and the frequency of using a cutting balloon was higher (26%) in the BTHC-PCB group than in the iopromide-PCB group (10%). Despite these limitations, it can be suggested that the clinical performance of both devices seems broadly comparable. Moreover, although the lack of a control group with plain angioplasty in bifurcation lesions might be seen as a gap in the evidence which does not allow full appreciation of the relative merits of DCB angioplasty in this setting, the clear evidence of the superiority of DCB from randomised trials means that specific studies of DCB versus plain balloon angioplasty in bifurcation restenosis are not likely to be performed in the future.
Finally, the use of KBA is generally recommended in bifurcation interventions, particularly if a two-stent approach is used1. However, in our study we did not observe an obvious association between KBA use and angiographic or clinical outcomes in this setting. However, the influence of treatment selection must be considered when interpreting these data as the proportion of true bifurcation lesions was about twice as high in the KBA group as in the no KBA group (66% versus 31%).
Our study has some important strengths. First, because we included all consecutive patients who underwent intervention with DCB for ISR at a bifurcation site with no exclusion criteria, our data may be representative of routine clinical practice in which DCB is used to treat ISR after bifurcation stenting across a spectrum of settings including acute coronary syndromes, left main bifurcation lesions, true bifurcation lesions and small side branches. Second, to the best of our knowledge, this is the largest study of angiographic outcomes in patients with ISR at a bifurcation and the first to use quantitative coronary angiography analysis. Third, we used an analysis protocol incorporating dedicated bifurcation analysis.
Limitations
Firstly, our findings represent a retrospective analysis of data from two enrolling centres in Germany. This limits the external validity of the data. Secondly, as already mentioned, findings in relation to the comparative efficacy of different treatments must be interpreted in the light of non-randomised treatment allocation and the unbalanced representation of left main bifurcations and cutting/scoring balloon angioplasty between treatment groups. Future randomised trials should focus on this knowledge gap. Thirdly, the influence of angiographic surveillance must be considered. This tends to result in higher rates of repeat revascularisation in comparison with patient cohorts without invasive surveillance. Fourthly, in a proportion of cases, DES or plain balloon angioplasty was also used in combination with DCB in the treatment of the bifurcation area. Specifically, although the proportion of treatment with DES was low, this must be borne in mind when interpreting the results. Finally, the influence of missing data should be considered. In particular, data on the type of stent technique used at baseline was only available in 81.9% of patients and angiographic surveillance data was available for only 70% of patients.
Conclusions
In the setting of ISR after coronary bifurcation stenting using a two-stent approach, angiographic and clinical outcomes following angioplasty with DCB were satisfactory. Moreover, similar outcomes were observed in patients treated with either the iopromide-PCB or the BTHC-PCB, and in patients who underwent final KBA versus those who did not.
| Impact on daily practice The incidence of in-stent restenosis after percutaneous intervention at coronary bifurcation sites remains considerable and the optimal management is not well defined. The present study shows satisfactory angiographic and one-year clinical outcomes following angioplasty with DCB for in-stent restenosis after coronary bifurcation stenting using a two-stent approach. On the basis of our data, angioplasty with DCB might be an attractive option for the management of in-stent restenosis after coronary bifurcation stenting. |
Conflict of interest statement
R. Colleran reports receiving support from the Irish Board for Training in Cardiovascular Medicine sponsored by MSD. A. Kastrati reports patent applications related to drug-eluting stent coatings. R. Byrne reports receiving lecture fees from B. Braun Melsungen AG, Biotronik and Boston Scientific, and institutional research grants from Boston Scientific and HeartFlow. The other authors have no conflicts of interest to declare.

