Abstract
Aims: We sought to describe the response of the polymer surface of drug-eluting stents (DES) to delivery balloon expansion, including quantitation of any resulting detached microparticles.
Methods and results: We expanded the US Food and Drug Administration (FDA)-approved first- and second-generation DES in a vacuum filtration system and used optical and scanning electron microscopy to image the polymer surface, filters and delivery balloons. DES were expanded under a range of conditions, from in vitro conditions used for FDA regulatory submissions to human in vivo conditions. Dispersive Raman spectroscopy was used for definitive identification of microparticles. All polymer surfaces were topographically disturbed over an average of 4.6%-100% of the surface area imaged. Disturbances ranged from deformation (including peeling) to complete delamination. The dimensions of detached microparticles were 2-350 μm. The extent and nature of surface disturbances and microparticles were primarily a function of polymer composition (p<0.001 for 8/10 disturbance types/locations) and were independent of expansion condition (p=0.100 to 0.989 for 9/10 disturbance types/locations).
Conclusions: Balloon expansion of first- and second-generation DES disturbs the polymer surface and can cause detachment of microparticles; each is functionally related to the specific polymer but not to expansion condition. Disturbance “roughness” and detached microparticles may contribute to DES limitations.
Introduction
Despite advances in prevention, pharmacotherapy and revascularisation, atherosclerotic coronary artery disease remains the leading cause of death in European and US adults1,2. Technical advances have positioned drug-eluting stents (DES) as the “state of the art” for revascularisation using percutaneous coronary intervention in the United States3. Compared with their predecessor bare metal stents (BMS), DES have reduced the incidence of restenosis in many subsets of patients4-9. However, DES have not eliminated restenosis, thrombotic events can occur early and late, and microvascular and endothelial dysfunction can develop during and after the DES index procedure10-16. Although the fundamental causes of these limitations have not been fully elucidated, each can result in life-threatening complications and/or further procedures for the patient10, adding to the already significant costs associated with DES (estimated at US $1.57 billion/year for Medicare in the USA)11,17.
Topographic disturbances to the polymer surface of DES during balloon expansion have been reported by two research groups18-21, giving rise to the hypothesis that such disturbances may lead to the detachment and embolisation of polymer fragments. Additionally, such detachment and embolisation of polymer fragments –and consequent ischaemic complications– have been described for other polymer-coated intravascular devices22. However, the sole published report linking polymer disturbances and microparticle detachment in DES consists of preliminary data from our own research group23. In this report, we describe more fully our methods and results in order to: 1) expand the knowledge base for disturbances to the polymer surface of DES caused by delivery balloon expansion, 2) quantitate the size of any resultant detached microparticles, and 3) argue that these factors should be measured and formally considered during future stent development.
Methods
DES/POLYMER PREPARATION AND EXPANSION
All four US Food and Drug Administration (FDA)-approved first- and second-generation DES were studied. The accompanying four polymers were: 1) parylene C base coat with a mixed polyethylene-co-vinyl acetate (PEVA)/poly n-butyl methacrylate (PBMA) drug-loaded layer and a top coat of PBMA (CYPHER® DES; Cordis, Johnson & Johnson, Warren, NJ, USA); 2) poly(styrene-b-isobutylene-b-styrene) drug-loaded coating (TAXUS® Liberté® DES; Boston Scientific, Natick, MA, USA); 3) poly n-butyl methacrylate base with a poly(vynilidine fluoride-co-hexafluoropropylene) drug-containing coating (XIENCE V® DES; Abbott Laboratories, Santa Clara, CA, USA); and 4) drug-loaded phosphorylcholine layer placed between phosphorylcholine top and base coats (Endeavor® DES; Medtronic, Minneapolis, MN, USA). DES diameters and lengths were 3.0 mm and 12-24 mm, respectively, and each DES tested originated from a different lot from the respective manufacturer. Each DES came directly from its original package, premounted on its delivery balloon by the manufacturer and not directly handled in any way.
Following optical microscopy (OM) examination of the polymer surface on the abluminal side of each DES in the unexpanded state, the accompanying delivery balloon was attached to a standard inflation system containing heparinised saline. Expansion of each DES was performed vertically in a vacuum filtration apparatus (Figure 1) containing a filtered test medium. The apparatus was loaded with inorganic membrane filters (Whatman Anapore; Springfield Mill, UK; membrane nominal pore size 20 nm for water, 200 nm for plasma) selected to allow spectroscopic identification of any particulate debris with minimal background contribution from the filter surface. All DES expansions and sample handling were performed in a Class II biological safety cabinet.
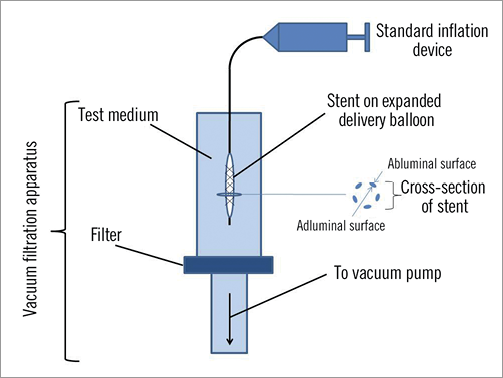
Figure 1. Schematic diagram of vacuum filtration apparatus.
Immediately prior to expansion, each DES was placed into the filtration apparatus and the accompanying delivery balloon was subjected to a negative pressure for 3-5 sec (simulating common clinical practice). The balloon was then inflated over a span of 10-15 sec to the final maximum inflation pressure. All delivery balloons remained inflated for 90 sec before being deflated by applying negative pressure to the inflation system. The DES was allowed to “drop off” the delivery balloon, which was then removed. A low-pressure vacuum (0.5 atm [50.7 kPa]) was applied to the filtration apparatus to draw the medium through the filter for particle recovery. Each stent, filter and balloon was then air-dried for microscopic examination.
This procedure was repeated to determine the response of each polymer to five selected expansion conditions, which comprised combinations of the maximum inflation pressure of the delivery balloon, the specific test medium, and the test medium’s temperature. Thus, five DES from each manufacturer were studied, one for each condition. The first three conditions spanned those from the in vitro conditions used for FDA regulatory submissions to the human in vivo condition. They involved a maximum inflation pressure of 14.0 atm (1,418.6 kPa) and test media that included deionised water at room temperature (25ºC), deionised water at body temperature (37ºC), and human plasma at body temperature. After a preliminary analysis of these three test conditions demonstrated no appreciable differences in topographic disturbance or microparticle detachment for the respective polymers, the subsequent two conditions utilised deionised water at room temperature with inflation pressures at clinical extremes of 22.0 atm (2,229.2 kPa) and 9.0 atm (911.9 kPa). For purposes of comparison, a Boston Scientific Liberté BMS (Boston Scientific, Natick, MA, USA) was expanded using the same technique under the first condition of a maximum inflation pressure of 14.0 atm in deionised water at room temperature.
OPTICAL AND SCANNING ELECTRON MICROSCOPY EXAMINATIONS
The adluminal and abluminal polymer surfaces of DES were systematically imaged following balloon expansion using OM (Olympus BX 60; Olympus America Inc., Center Valley, PA, USA). Magnification was 50-500×. Each polymer surface was imaged at 16 locations (eight adluminal, eight abluminal) spanning the length of the DES. The locations were predefined in a spiral configuration. For quantitative measurements, the magnification was 100×. The initial location was <1 mm on the trailing side of the leading edge of the DES. Each successive location was 1-2 mm further down the trailing side of the DES, and the DES was rotated 45º for each successive location. Topographic disturbances were categorised as deformation of the surface (ridging, cracking, and peeling) and complete delamination23. Additionally, any obstruction of open cells by the polymer (webbing) was recorded. The proportion of the surface area affected by each form of disturbance relative to the total surface area imaged was then determined using quantitative image analysis. For webbing, the area of the two bases forming each individual web was used for the calculation. Scanning electron microscopy (SEM; JEOL JSM6335F; JEOL Ltd., Tokyo, Japan) at a magnification of 25-1,000× was used to characterise polymer surfaces of DES at discrete locations. Similar imaging was performed for the BMS and for the abluminal polymer surface of each DES prior to expansion.
Filter and delivery balloon surfaces were imaged using both OM and SEM. Dispersive Raman spectroscopy (Renishaw inVia Raman microscope; Renishaw PLC, New Mills, Wotton-under-Edge, Gloucestershire, UK) was used for definitive identification of any microparticles on filters and delivery balloons. Particle size distribution was determined using image analysis for any identified microparticles recovered on filters (ImagePro Plus version 6.2; Media Cybernetics, Bethesda, MD, USA).
STATISTICAL ANALYSIS
The mean (SD) of the proportion of the stent surface topographically disturbed, categorised by type of disturbance (ridging, cracking, peeling, delamination, or webbing) and location (adluminal vs. abluminal) averaged over condition for each DES, was computed. To test whether the proportion of damage differed by specific DES or condition, we employed generalised linear mixed models. The log odds of the proportion of the surface disturbed at each image location were allowed to vary as a function of the DES and condition, and binomial type variance was assumed. Because of repeated images taken on each DES, a random effect for each DES was included. Type 3 F-tests were used to determine whether these factors (DES polymer and condition) were significant. Separate models were constructed for each type of disturbance and location. A similar modelling approach was used to test whether the proportion of total disturbance differed by location for each DES. Alpha levels ≤0.05 were considered statistically significant and were two-sided. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
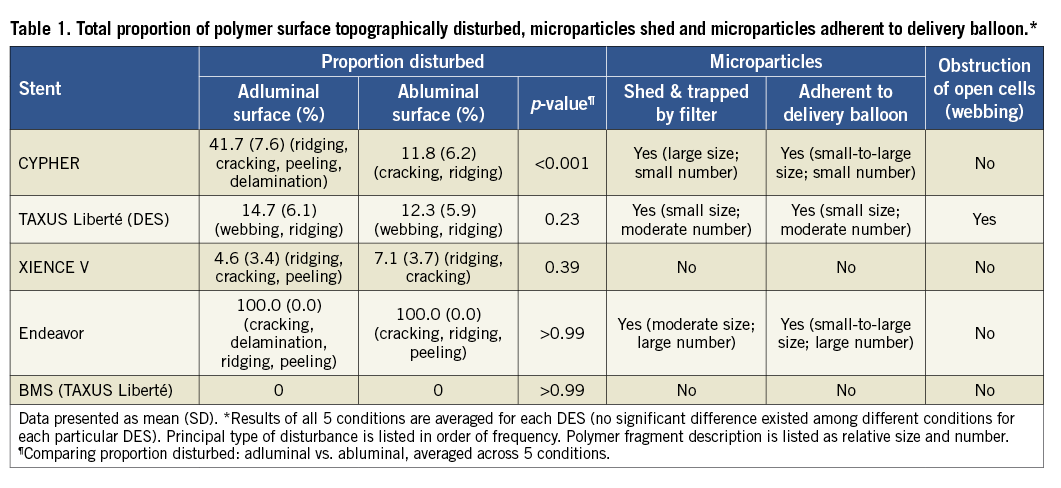
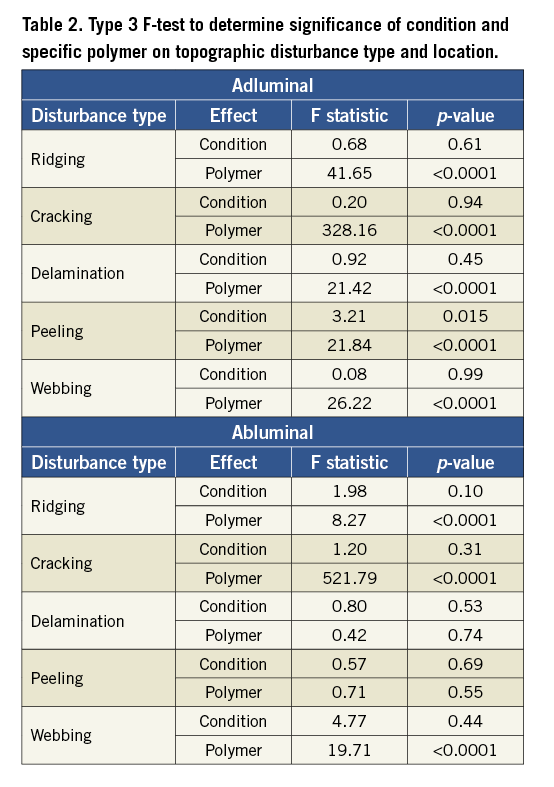
The adluminal polymer surface of each DES was topographically disturbed, affecting 4.6% to 100% of the surface area imaged (Table 1). Expansion condition did not significantly affect disturbance (Table 2). Figure 2 shows representative disturbance to the different polymers under the first condition. The average proportion of the surface disturbed as a function of type of disturbance, location and specific DES is shown in Figure 3. Ridging was most common with the CYPHER DES, affecting 33.0% (8.5%) of the adluminal surface, compared with 2.1%-4.7% for other DES (p<0.001 for group test effect of polymer on proportion ridging). Although less frequent, peeling was also most common with the CYPHER, affecting 2.7% (2.9%) of the adluminal surface, compared with 0.02%-0.35% for other DES (p<0.001). Raman spectroscopy showed that the ridging, peeling (and delamination) visualised on the CYPHER primarily involved the top two coating layers (the drug-containing PEVA/PBMA polymer layer and the drug-free PBMA top coat), leaving the parylene C polymer base coat intact. Cracking was most common with the Endeavor DES, affecting 76.8% (8.3%) of the adluminal surface compared with 0.03%-3.5% for the other DES (p<0.001). Similarly, delamination was most common with the Endeavor, affecting 18.5% (7.6%) of the adluminal surface, compared with 0.04%-2.5% for the other DES (p<0.001). Webbing occurred almost exclusively with the TAXUS Liberté DES (12.4% [6.5%] of adluminal surface; p<0.001). The XIENCE V DES was least disturbed.
The abluminal polymer surface of each respective DES was also disturbed, with dimensions similar to the adluminal surface. The proportions of the surface area and the nature of the abluminal disturbances were similar to the adluminal disturbances for the TAXUS Liberté and XIENCE V (p=0.23 and 0.39, respectively, for comparison of total disturbance) (Table 1, Figure 3). However, the extent of abluminal surface area disturbance was significantly less for the CYPHER (p<0.001), primarily as a result of less ridging. Although there was less delamination on the abluminal surface of the Endeavor, there was more cracking. Importantly, the abluminal surface of each DES prior to expansion did not exhibit any significant disturbance, with the exception of the Endeavor, which showed cracking (Figure 2). All surfaces of control BMS were essentially undisturbed, showing only minor surface indentations.
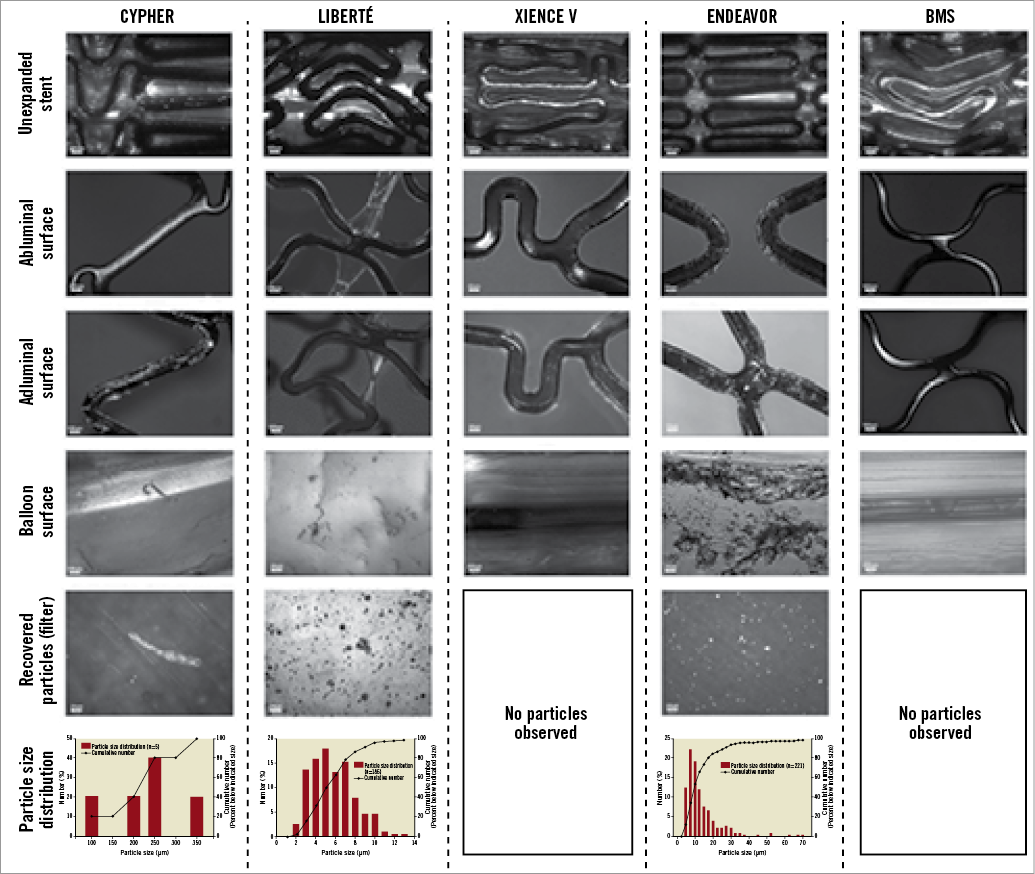
Figure 2. Representative topographical disturbance to the different polymers, microparticles shed, and microparticles adherent to delivery balloon for each DES and BMS under first condition. Each stent shown was 3.0 mm in diameter and 16-18 mm in length. CYPHER DES: areas of ridging, cracking, peeling and delamination can be easily seen on the adluminal surface. In addition, large microparticles can be easily seen adherent to the delivery balloon and on the filter. TAXUS Liberté DES: areas of webbing and ridging can be easily seen on the adluminal and abluminal surfaces, as can small microparticles adherent to the delivery balloon and on the filter. XIENCE V DES: cracking and peeling can be seen on the adluminal surface. No microparticles were identified on the balloon surface or filter. Endeavor DES: the pre-expanded DES demonstrates extensive cracking. In addition, cracking, ridging and delamination can be easily seen universally on the adluminal surface of the expanded DES. However, while the abluminal surface demonstrates cracking, peeling and ridging, there is no apparent delamination. The BMS demonstrates minor surface indentations but no other disturbance or microparticles.
Examination of filters showed that each DES, except the XIENCE V, shed microparticles into the test medium. Detached microparticles ranged in size from 2-350 μm. The Endeavor shed the greatest number of microparticles, which were small (4-70 μm) (Figure 2). The TAXUS Liberté also shed a significant number of relatively smaller microparticles; the CYPHER shed few but relatively large microparticles. Raman spectroscopy showed that the CYPHER microparticles were composed of the drug-containing PEVA/PBMA polymer and the drug-free PBMA top coat. The BMS did not shed detectible microparticles. The number and dimensions of the microparticles adhering to the delivery balloons were similar to microparticles shed for each respective DES, with none detected on the XIENCE V and BMS delivery balloons.
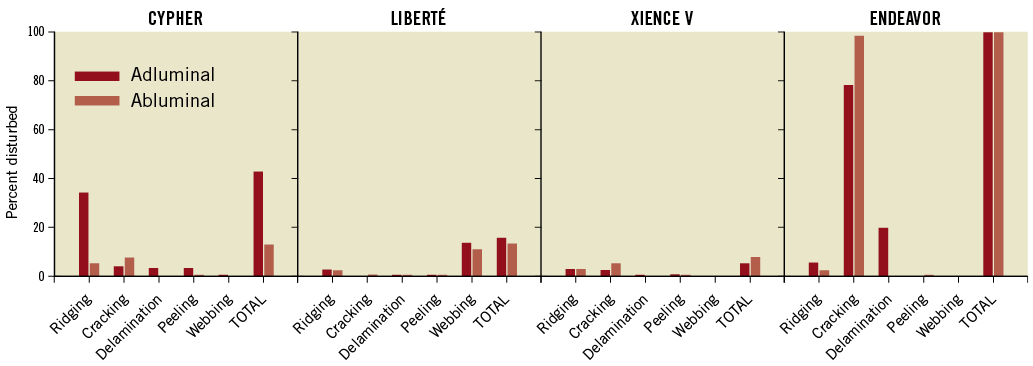
Figure 3. Average proportion of polymer surface topographically disturbed as a function of type of disturbance, location, and specific DES.
The proportions of the surface area and nature of disturbances, number and size of microparticles shed, and number and size of microparticles adherent to the balloon were primarily a function of polymer composition and, as above, were statistically unrelated to expansion condition (Table 2).
Discussion
Our study confirms that expansion of the delivery balloon topographically disturbs the polymer surfaces of FDA-approved first- and second-generation DES, and that this disturbance can be complicated by the detachment of microparticles. Additionally, we showed that the disturbance to the polymer involves both the adluminal and abluminal surfaces and that detached microparticles can be shed into the test medium (and are therefore susceptible to embolisation in vivo). Finally, we showed that the extent and nature of polymer disturbance and detached microparticles are primarily a function of polymer composition and are unrelated to expansion condition.
BEHAVIOUR OF POLYMER DURING DELIVERY BALLOON EXPANSION
The behaviour of the polymer surface of DES during delivery balloon expansion has been examined by only two research groups in the peer-reviewed literature18-21, and our preliminary report is the only one to date that has focused attention on the topic of the potential detachment of microparticles23. Early research into polymers for use in drug elution concentrated on diffusion from a plane sheet24 and then diffusion from membranes with a slab geometry25. Subsequent preliminary research into DES assumed insignificant spatial distribution effects and neglected the effect of forces that accompany balloon expansion on the polymer surface26. The few contemporary peer-reviewed studies that address the effect of balloon expansion have focused solely on the polymer surface and have not objectively addressed the possibility of detachment of microparticles18-21. Recent non-peer-reviewed studies performed by industry for FDA device approval have addressed the possibility of detached microparticles from third-generation DES, but only qualitative summaries are provided27,28.
The disturbance to the adluminal polymer surface and the complicating detachment of microparticles can be anticipated due to the adluminal polymer’s direct contact with the delivery balloon and the radial and shear forces created during balloon expansion29. However, this mechanism cannot explain the observed disturbance to the abluminal surface. A feasible alternative mechanism would depend on the continuous nature of the polymer from adluminal to abluminal surface and a transmission of force during balloon expansion along this surface30. In addition, there can be a significant mismatch in the elongation at break for the ductile metal of the stent and the polymer coating, leading to significant shear stress at the polymer-metal interface during expansion31.
Unfortunately, the results of expanding DES in different conditions indicate that any lubricating effect of plasma and increased elasticity of the polymers at body temperature do not significantly affect the extent or nature of disturbance for the adluminal or abluminal polymer surfaces we examined, nor do they affect detachment of microparticles. However, the results of expanding DES in different conditions do suggest that in vitro studies using a test medium of deionised water at room temperature may adequately simulate the human in vivo condition for the polymers studied here. To our knowledge, this observation has never been documented but is assumed by the FDA32.
DES: RESTENOSIS AND THROMBOSIS
The topographic disturbance to the polymers we observed may help to explain the large concentration gradients of drug that have been measured over few microns of expanded DES, with relatively minor effects on overall mean concentration33. Explanations of this phenomenon previously focused on drug physiochemical properties and expanded stent configuration. However, the different types of polymer disturbance we observed can, independently of drug properties and stent configuration, lead to areas of small (e.g., delamination) or large (e.g., ridging) concentrations of drug. This would limit anticipated retardation of neointimal hyperplasia and cause delay in arterial healing, respectively, in turn resulting in a predisposition for restenosis and late thrombosis34,35. Although stent polymer has been identified as a causative factor in late thrombosis through an inflammatory response36, it has not been implicated in thrombosis or restenosis by means of a mechanism such as inhomogeneity of drug distribution following disturbance from balloon expansion. This mechanism could also explain the topographic inhomogeneity of restenosis observed within DES using optical coherence tomography37.
The topographic surface irregularities caused by polymer disturbance (e.g., areas of peeling; transition zones into areas of delamination; webbing) could contribute to platelet adhesion and consequent early and late thrombosis, especially with “rougher” surfaces38,39. In addition, obstruction of open cells by webbing of the polymer and resultant side-branch obstruction could contribute directly to periprocedural acute myocardial infarction (MI)7. DES thrombosis and restenosis seem to be device-specific (with meta-analyses favouring XIENCE V)40,41, and our results suggest that topographic disturbance to the polymer should be investigated as a direct contributing cause to these clinically relevant phenomena.
DES: MICROVASCULAR AND ENDOTHELIAL DYSFUNCTION
Microparticles (including drug) shed following topographic disturbance of the polymer may also potentially contribute to restenosis, thrombosis and other adverse events (e.g., periprocedural acute MI) through microvascular obstruction, endothelial dysfunction and impaired coronary vasomotion42,43. The immediate effect of the microparticles per se would be mechanical obstruction; however, the immediate effect of any accompanying drug would be chemical and potentially toxic32. Case reports of embolisation of polymer fragments from other polymer-coated intravascular devices have been described and correlated with subsequent adverse clinical events22, but the sole published report that has focused on the possibility of DES microparticle embolisation involves our own preliminary data23.
Endothelial dysfunction and impaired coronary vasomotion following DES implantation seem to be device-specific (CYPHER and TAXUS more so than Endeavor; not described for XIENCE V)14,15. Similarly, periprocedural acute MI seems to be device-specific (occurring least frequently with XIENCE V)44. Our results suggest microparticle detachment and embolisation should be investigated as a contributing cause of these clinically relevant phenomena via microvascular obstruction and endothelial dysfunction.
Limitations
This study expanded DES within a vacuum filtration apparatus offering essentially zero resistance and no contact surface (e.g., arterial wall) against the abluminal polymer surface. The in vivo advancement of a DES through the coronary circulation may topographically disturb the abluminal polymer surface45, as could its expansion against an arterial wall; further, the expansion against an arterial wall could, through surface transmission of force, affect the adluminal polymer surface30. Thus, this study may underestimate the severity of disturbance to the polymer compared with the in vivo condition. Conversely, although microparticles may be generated in conjunction with topographic disturbances induced during advancement of the DES through the coronary circulation, the expansion of the DES within an artery may decrease embolisation of microparticles by confining (or “trapping”) them between the expanded abluminal surface of the DES and the arterial wall. Thus, our study may contain a systematic error with regard to determining an absolute number of microparticles detached, and we have therefore omitted that result. However, such an error would not affect our qualitative assessment of the relative number of microparticles detached. Finally, translating the results of this study to clinical trials is confounded by other components of DES - namely, the drug and stent superstructure - which may also affect the results of this study and clinical trials.
Conclusions
Delivery balloon expansion of first- and second-generation DES topographically disturbs the polymer surface and can cause detachment of microparticles which can embolise in vivo. Each is functionally related to the specific polymer but not to expansion condition. The contribution of these effects to restenosis, thrombosis, and microvascular and endothelial dysfunction should be pursued through coordinated physiological and clinical studies. Additionally, in accordance with the spirit of the European Medicines Agency (EMA) guidelines46 and the FDA DES Draft Guidance for Industry32, more specific and quantitative results of polymer disturbance and microparticle detachment from manufactures of first-, second-, and third-generation DES should be made available to practising interventional cardiologists. These measures together could translate into improved patient outcomes and decreased costs.
Acknowledgements
The authors wish to thank Bram Zuckerman, MD, Ashley Boam, MSBE, and Andrew Farb, MD, of the Center for Devices and Radiological Health, FDA, Rockville, MD, USA, for sharing their insights and for reviewing the preliminary manuscript. The authors also thank Jonathan McCall, MS, for providing editorial assistance with this article as part of his regular duties as a medical editor employed by the Duke Clinical Research Institute, Durham, NC, USA.
Funding
University of Florida Gatorade Fund.
Conflict of interest statement
Harry R. Phillips III, MD: ownership interest; significant; Abbott Laboratories. The other authors have no conflicts of interest to declare.

