
Abstract
Aims: Several studies have suggested good procedural and similar clinical outcomes between everolimus-eluting Absorb bioresorbable stents (BRS) versus conventional drug-eluting stents (DES), but the evidence is not definitive. Our aim was to perform a systematic review and meta-analysis to investigate the effects of BRS versus conventional drug-eluting and bare metallic stents on the cardiovascular endpoints and all-cause mortality.
Methods and results: The follow-up in the included studies was up to 13 months. The following endpoints were evaluated: all-cause mortality, cardiac death, patient-oriented major adverse cardiac events (POCE), device-oriented major adverse cardiac events (DOCE), any-cause myocardial infarction (MI), target vessel MI (TVMI), target vessel revascularisation (TVR) and target lesion revascularisation (TLR). The results of 10 studies with 5,773 subjects showed a statistically significant increase in the risk of TVMI between BRS and conventional stents (odds ratio [OR]: 1.45, 95% confidence interval [CI]: 1.03-2.05, p=0.032). None of the other differences reached statistical significance: all-cause mortality (OR: 0.67, 95% CI: 0.30-1.49, p=0.333), cardiac death (OR: 1.00, 95% CI: 0.47-2.12, p=0.996), POCE (OR: 0.91, 95% CI: 0.68-1.22, p=0.546), DOCE (OR: 1.12, 95% CI: 0.86-1.46, p=0.387), any-cause MI (OR: 1.34, 95% CI: 0.98-1.82, p=0.064), TVR (OR: 0.99, 95% CI: 0.73-1.33, p=0.934) and TLR (OR: 0.92, 95% CI: 0.66-1.29, p=0.641). Similar results were observed after restricting the meta-analysis to the comparison of BRS vs. EES.
Conclusions: Our meta-analysis suggests a significantly higher risk of TVMI with BRS compared with conventional stents and no significant differences in the rates of occurrence of the other outcomes during one-year follow-up. Further studies with larger samples sizes, longer follow-up, different clinical scenarios and more complex lesions are required to confirm or refute our findings.
Abbreviations
BMI: body mass index
BRS: bioresorbable stents
BVS: bioresorbable vascular scaffold
DES: drug-eluting stents
DOCE: device-oriented major adverse cardiac events
EAPCI: European Association of Percutaneous Coronary Interventions
EES: everolimus-eluting stent
ESC: European Society of Cardiology
MI: myocardial infarction
MRI: magnetic resonance imaging
OCT: optical coherence tomography
POCE: patient-oriented major adverse cardiac events
STEMI: ST-elevation myocardial infarction
TLR: target lesion revascularisation
TVMI: target vessel myocardial infarction
TVR: target vessel revascularisation
Introduction
In the last few dozen years, significant innovations1 have been introduced in the stent technology used in percutaneous coronary intervention (PCI)1, starting with bare metal stents (BMS), followed by metallic drug-eluting stents (DES) and culminating with the appearance of the Absorb (Abbott Vascular, Santa Clara, CA, USA) bioresorbable vascular scaffold (BVS), considered as the fourth revolution in interventional cardiology2,3. The industry of medical devices followed two strategies in BVS development, one based on magnesium-based scaffolds, and the other on backbones composed of lactic acid polymers4. Although no BVS has yet been authorised in the USA, two lactic-acid polymer devices, the everolimus-eluting Absorb stent and the novolimus-eluting DESolve® stent (Elixir Medical Corporation, Sunnyvale, CA, USA), have received approval in Europe5. Recently, the European Society of Cardiology (ESC)/European Association of Percutaneous Coronary Interventions (EAPCI) task force on the evaluation of coronary stents in Europe6 agreed that bioresorbable stents (BRS) is a more suitable term for BVS, since a scaffold might indicate a need for a temporary arterial support7. Since their availability on the European market in November 2011, an impressive evolution towards more than 60,000 implanted BRS had the purpose of addressing the most significant pathophysiological complications observed in previous metallic DES, such as limitation of vascular function, long-term flow disturbance, a chronic inflammatory state, the risk of neo-intimalisation or late and very late stent thrombosis8. BRS disappear in two to four years after implantation (usually about two years), to restore the normal structure and physiology of the vessels, to support complete vascular healing, to restore shear stress, to reduce thrombotic propensity and the optimal duration of dual antiplatelet therapy, and even to improve the usefulness of coronary imaging using multislice computed tomography (CT) and magnetic resonance imaging (MRI) without artefacts2. Moreover, BRS are currently being evaluated as a new solution for acute coronary syndromes with ST-elevation myocardial infarction (STEMI)9,10, advanced peripheral artery disease11, or coronary ostial lesions12 due to their demonstrated ability to permit recovery of the normal geometry of arteries13.
BRS might provide attractive physiologic advancements over the existing drug-eluting or metallic stents, but the evidence is still not definitive. Consequently, we performed a systematic review and meta-analysis to investigate the effects of BRS versus drug-eluting and metallic stents on at least one clinical endpoint including all-cause mortality, cardiac death, patient-oriented major adverse cardiac events (POCE; including death, non-fatal vessel-related myocardial infarction [MI] and target lesion revascularisation [TLR]), device-oriented major adverse cardiac events (DOCE; including death, non-fatal vessel-related MI, TLR), any-cause MI, target vessel MI (TVMI), target vessel revascularisation (TVR) and TLR.
Methods
SEARCH STRATEGY
The study was designed according to the guidelines of the 2009 preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement14. PubMed/Medline, SCOPUS, and Google Scholar databases were searched using the following search terms in titles and abstracts: Bioresorbable Vascular Scaffold OR BVS OR Bioresorbable Stents OR BRS OR Bioresorbable Scaffold OR ABSORB AND drug-eluting stents (DES) OR everolimus OR Everolimus-Eluting Stents OR everolimus-eluting OR everolimus-eluting stents OR Zotarolimus OR Biolimus OR Biolimus-Eluting OR Biolimus-Eluting Stents. Additional searches for potential trials included the references of review BRS articles, and the following congresses: scientific sessions of the European Society of Cardiology (ESC), the American Heart Association (AHA), the American College of Cardiology (ACC), EuroPCR, and Transcatheter Cardiovascular Therapeutics (TCT). The wild-card term ‘‘*’’ was used to increase the sensitivity of the search strategy. The search was limited to articles published in the English language. Two reviewers (M.C. Serban and A. Sahebkar) examined every article separately to minimise the possibility of duplication, investigating reviews, case studies and experimental studies. Disagreements were managed by discussion with a third party (M. Banach). The literature was searched from inception to 15 October 2015.
STUDY SELECTION
The following criteria were used to identify eligible studies: (i) investigating the effects of bioresorbable stents versus drug-eluting stents or bare metallic stents, (ii) providing information on at least one clinical endpoint including all-cause mortality, cardiac death, POCE, DOCE, any-cause MI, TVMI, TVR and TLR.
Exclusion criteria were: (i) experimental studies, (ii) uncontrolled single-arm studies, and (iii) lack of assessment of clinical outcomes. Single-arm studies with comparator control arms from other studies were only included if matching was performed between the stent groups from different studies. In this latter case, the possibility of common treatment arms among the included studies was assessed first to rule out double counting of individuals.
DATA EXTRACTION
Eligible studies were reviewed and the following data were abstracted: 1) first author’s name; 2) year of publication; 3) country where the study was performed; 4) study design; 5) number of participants in the study groups; 6) type of stent; 7) duration of follow-up; 8) underlying disease; 9) age, gender and body mass index (BMI) of study participants; and 10) incidence of clinical outcomes.
Quality assessment
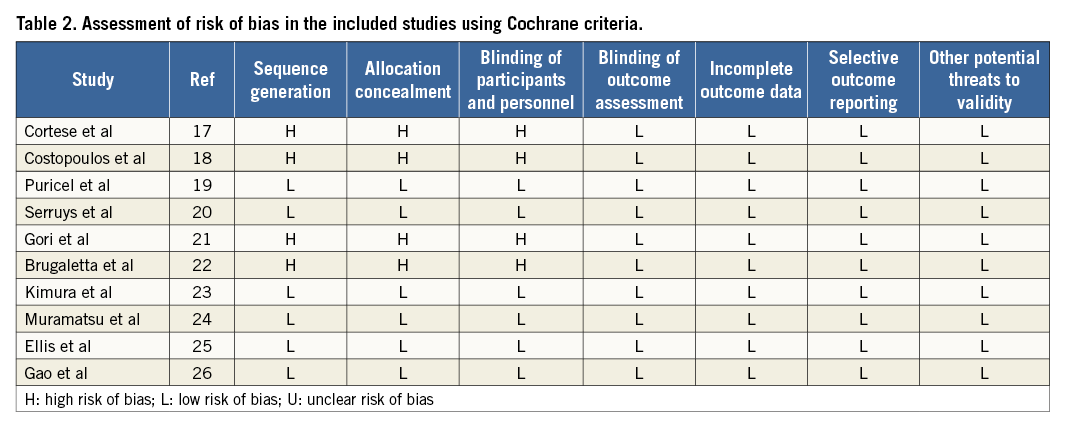
A systematic assessment of bias in the included studies was performed using the Cochrane criteria15. The items used for the assessment of each study were as follows: adequacy of sequence generation, allocation concealment, blinding, addressing of dropouts (incomplete outcome data), selective outcome reporting, and other potential sources of bias. According to the recommendations of the Cochrane Handbook, a judgement of “yes” indicated a low risk of bias, while “no” indicated a high risk of bias. Labelling an item as “unclear” indicated an unclear or unknown risk of bias.
Quantitative data synthesis
This meta-analysis was conducted using Comprehensive Meta-Analysis (CMA) V2 software (Biostat, Englewood, NJ, USA)16. Odds ratios (ORs) of clinical outcomes and 95% confidence intervals (without zero cell correction) were calculated as summary statistics and displayed as forest plots. Heterogeneity was quantitatively assessed using the I2 index, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance. Meta-analysis was performed using a random-effects (DerSimonian-Laird method) model. Due to the relatively small number of events in some of the studies and low indicators of heterogeneity, as a supporting analysis we also employed the fixed-effect Mantel-Haenszel method. In order to evaluate the influence of each study on the overall effect size, sensitivity analyses were conducted using the one-study remove approach. A nominal level of 0.05 was used to declare statistical significance; given nine outcomes considered, the Bonferroni-corrected level of significance would be 0.0056.
Results
The initial screening for possible interpretation eliminated the articles whose titles and/or abstracts were clearly insignificant. After evaluation, 10 studies met the inclusion criteria and were chosen for the final meta-analysis: eight had two treatment arms comparing an everolimus-eluting bioresorbable scaffold (Absorb) vs. an everolimus-eluting stent (EES) (XIENCE; Abbott Vascular), one study had three arms – an everolimus-eluting bioresorbable scaffold (Absorb) vs. an everolimus-eluting stent (XIENCE) vs. a biolimus-eluting stent (Biosensors Europe SA, Morges, Switzerland), and another study had three arms – an everolimus-eluting bioresorbable scaffold (Absorb) vs. an everolimus-eluting stent (XIENCE) vs. a bare metal stent (BMS) (MULTI-LINK VISION®; Abbott Vascular)17-26. A study flow chart is presented in Figure 1.
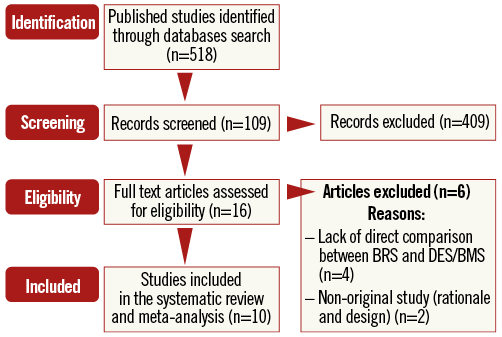
Figure 1. Flow chart of the number of studies identified and included in the meta-analysis.
Characteristics of included studies
Ten studies with a total population of 5,773 subjects were included, of whom 3,000 were assigned to the BRS group, 2,483 to the drug-eluting stent group, and 290 to the bare metal stent group. The number of participants in these trials ranged from 184 to 2,008. Studies were published between 2014 and 2015, and were conducted in Italy, USA, China, Switzerland, United Kingdom, Netherlands, France and Japan. Duration of follow-up ranged from six to 13 months. Demographic and baseline parameters of the included studies are shown in Table 1.
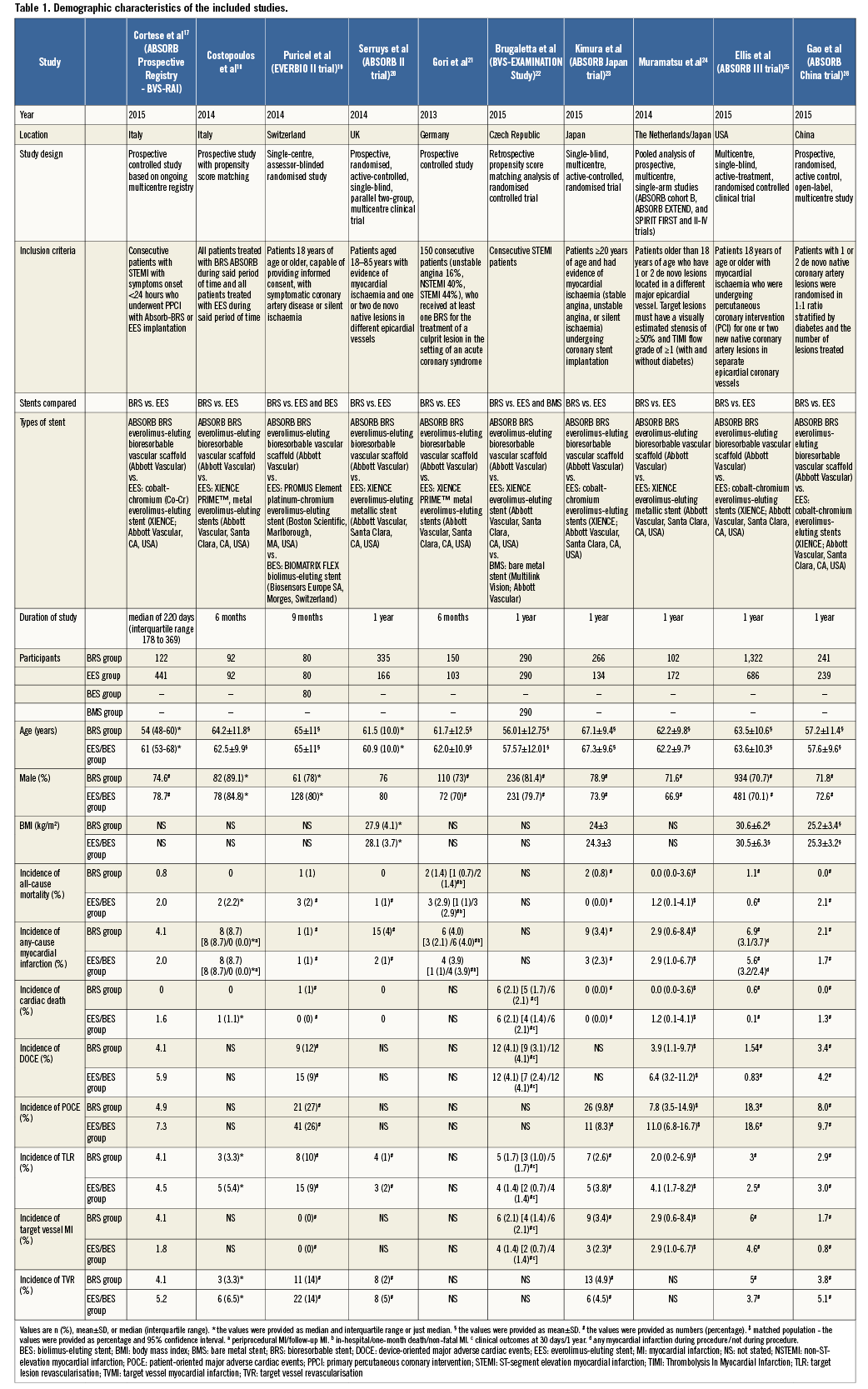
Risk of bias assessment
Details of the quality of bias assessment are shown in Table 2.
Effect of BRS versus conventional stents on clinical outcomes
Overall, the impact of BRS versus conventional stents on all-cause mortality, cardiac death, POCE, DOCE, any-cause MI, TVMI, TVR and TLR were reported by 9, 9, 6, 7, 7, 7, 7 and 9 studies, respectively. The difference between BRS and non-BRS (including DES and BMS) stents in all-cause mortality was not statistically significant (odds ratio [OR]: 0.67, 95% confidence interval [CI]: 0.3-1.49, p=0.333) and the I2 of 11.5% suggested little concern about the heterogeneity of individual study results (Figure 2). The results for cardiac death were also non-significant (OR: 1.00, 95% CI: 0.47-2.12, p=0.996; I2: 0%) (Figure 3). For any-cause MI the numerically large point estimate also did not reach statistical significance. We observed a significantly higher frequency of TVMI with BRS (OR: 1.45, 95% CI: 1.03-2.05, p=0.032; I2: 0%) (Figure 4). This translated into a numerically higher risk of any-cause MI, which did not reach statistical significance in the primary random-effects model (OR: 1.34, 95% CI: 0.98-1.82, p=0.064; I2: 0%) (Figure 5) but became statistically significant when analysed using the Mantel-Haenszel method (OR 1.36, 95% CI: 1.00-1.85, p=0.049). All other results were non-significant: POCE (OR: 0.91, 95% CI: 0.68-1.22, p=0.546; I2: 0%) (Figure 6), DOCE (OR: 1.12, 95% CI: 0.86-1.46, p=0.387; I2: 0%) (Figure 7), TVR (OR: 0.99, 95% CI: 0.73-1.33, p=0.934; I2: 0%) (Figure 8), and TLR (OR: 0.92, 95% CI: 0.66-1.29, p=0.641; I2: 0%) (Figure 9).
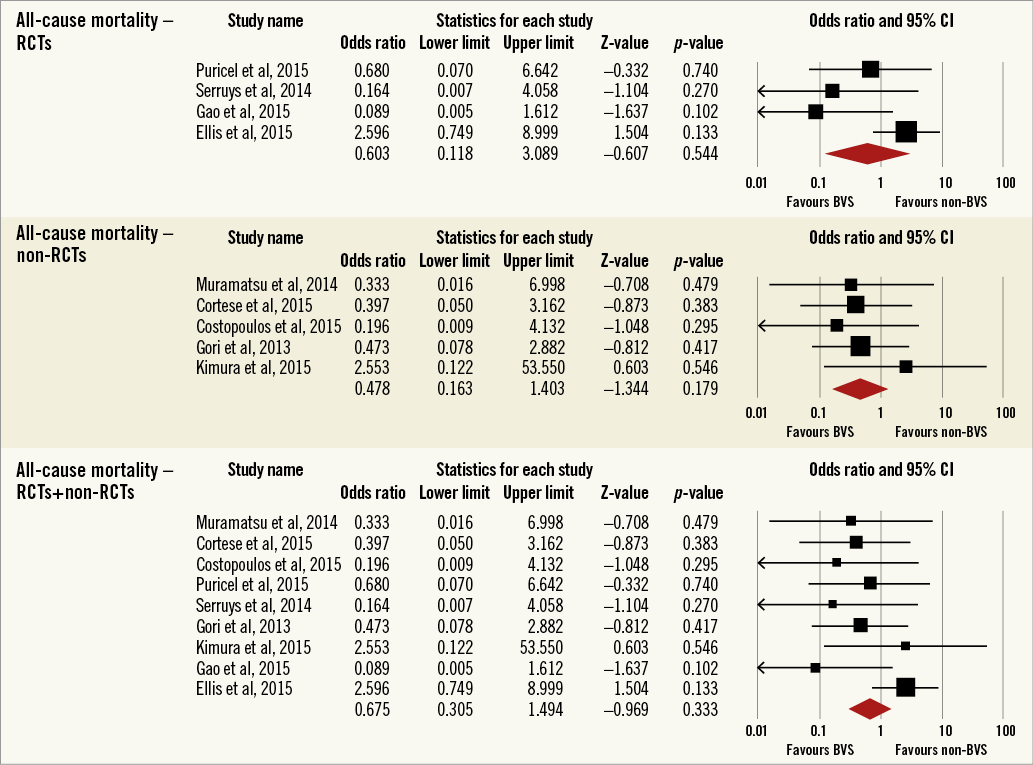
Figure 2. Forest plot displaying odds ratios (ORs) and 95% confidence intervals (CIs) for all-cause mortality in patients treated with bioresorbable vascular stent versus conventional stents.
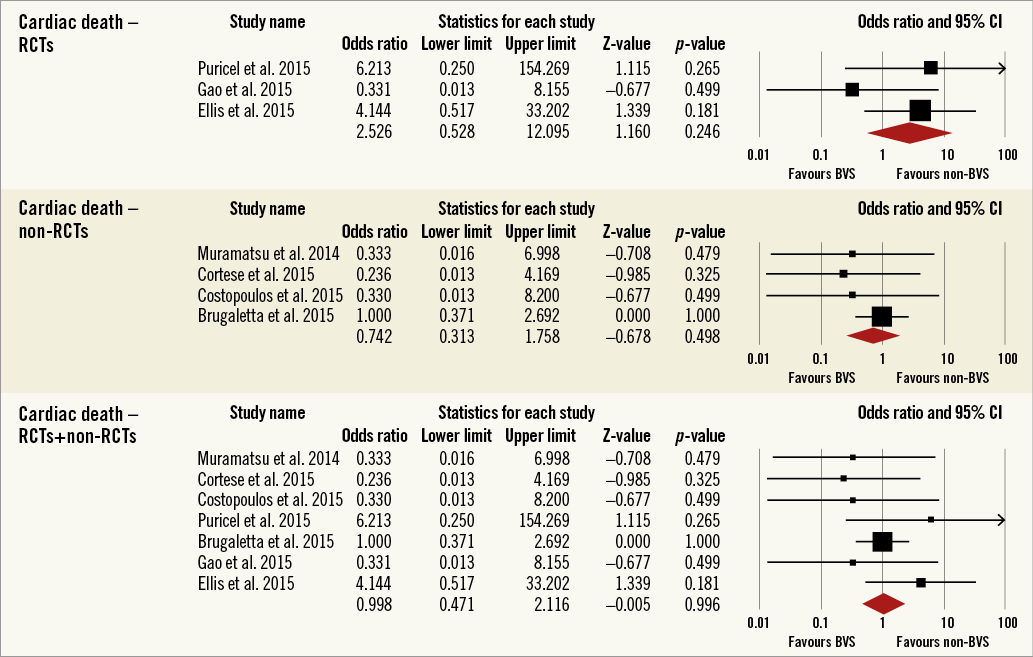
Figure 3. Forest plot displaying odds ratios (ORs) and 95% confidence intervals (CIs) for cardiac death in patients treated with bioresorbable vascular stent versus conventional stents.
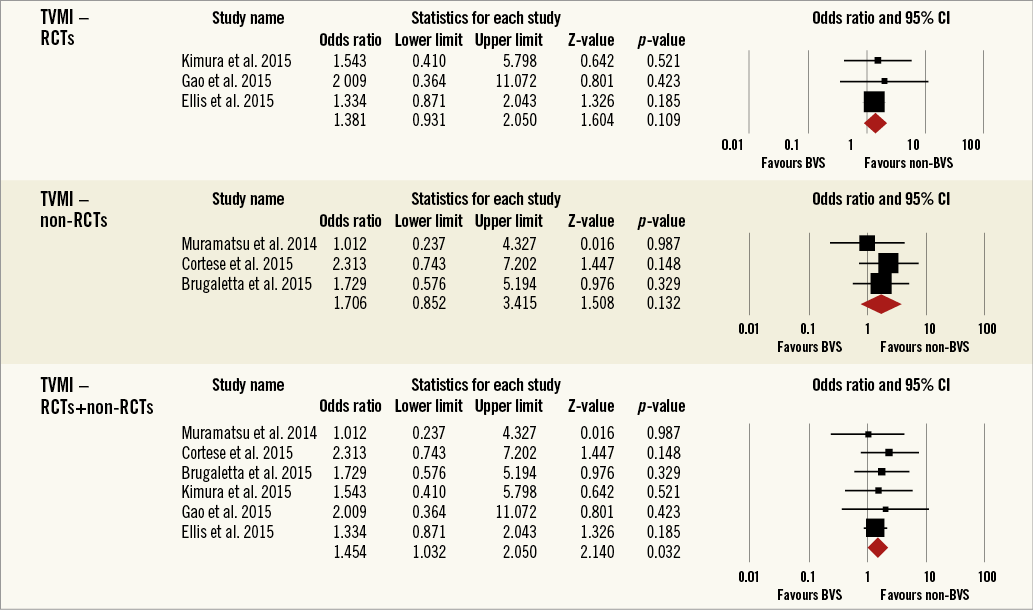
Figure 4. Forest plot displaying odds ratios (ORs) and 95% confidence intervals (CIs) for target vessel myocardial infarction in patients treated with bioresorbable vascular stent versus conventional stents.
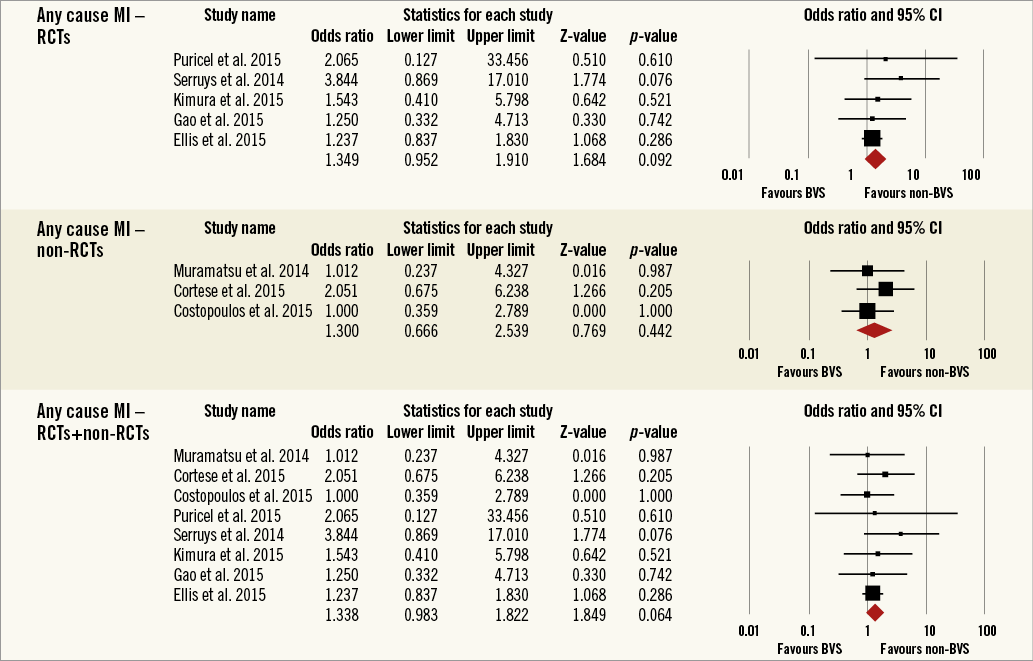
Figure 5. Forest plot displaying odds ratios (ORs) and 95% confidence intervals (CIs) for any myocardial infarction in patients treated with bioresorbable vascular stent versus conventional stents.
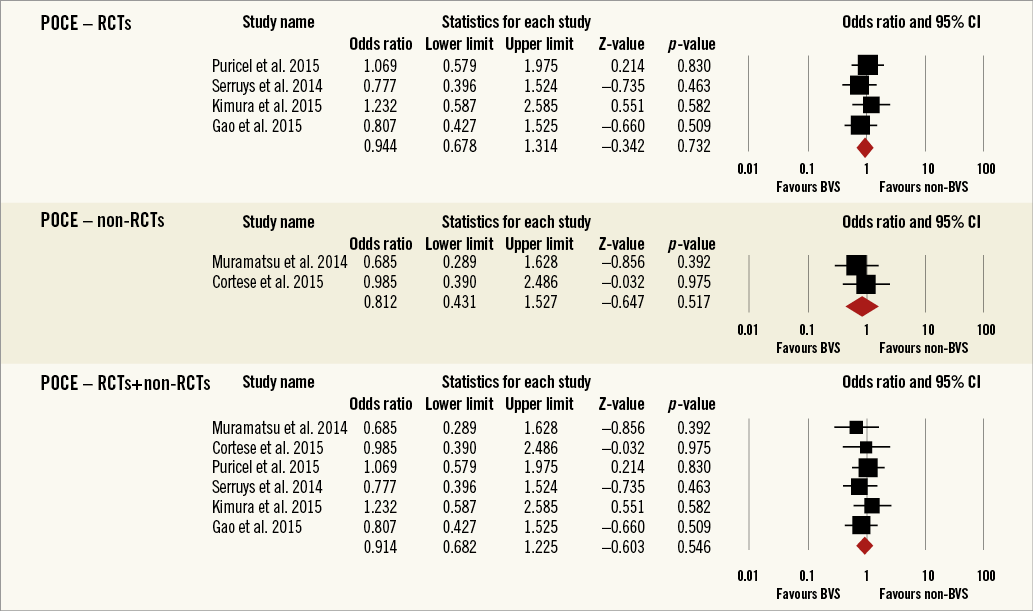
Figure 6. Forest plot displaying odds ratios (ORs) and 95% confidence intervals (CIs) for patient-oriented major adverse cardiac events in patients treated with bioresorbable vascular stent versus conventional stents.
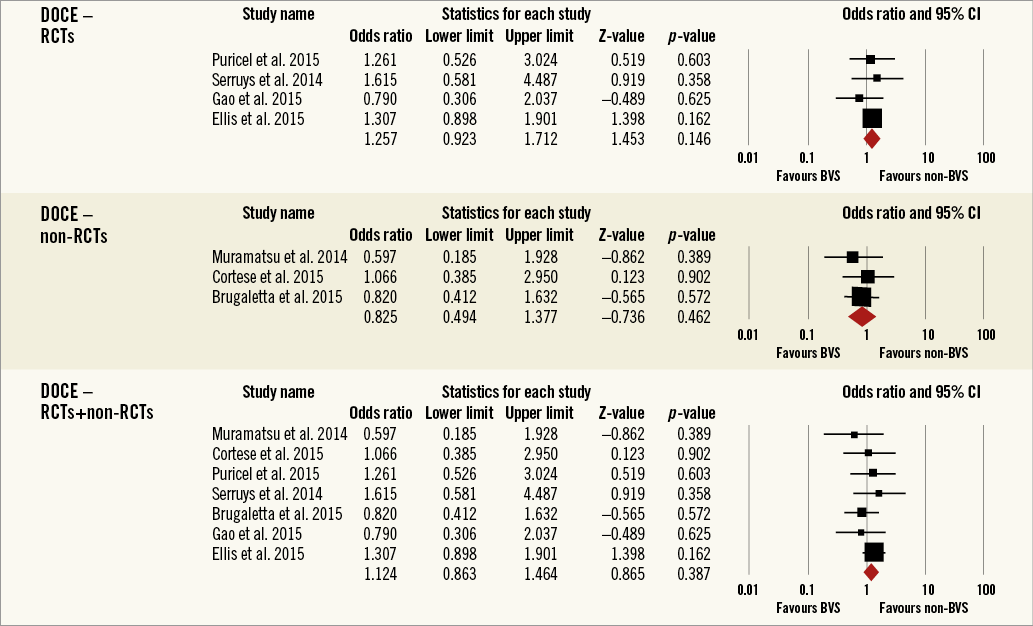
Figure 7. Forest plot displaying odds ratios (ORs) and 95% confidence intervals (CIs) for device-oriented major adverse cardiac events in patients treated with bioresorbable vascular stent versus conventional stents.
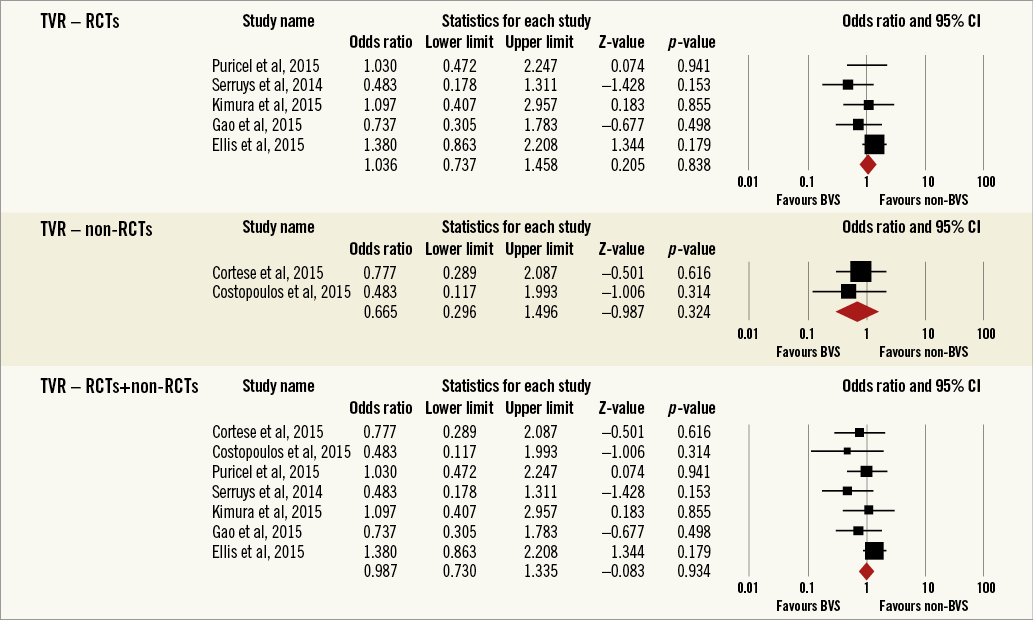
Figure 8. Forest plot displaying odds ratios (ORs) and 95% confidence intervals (CIs) for target vessel revascularisation in patients treated with bioresorbable vascular stent versus conventional stents.
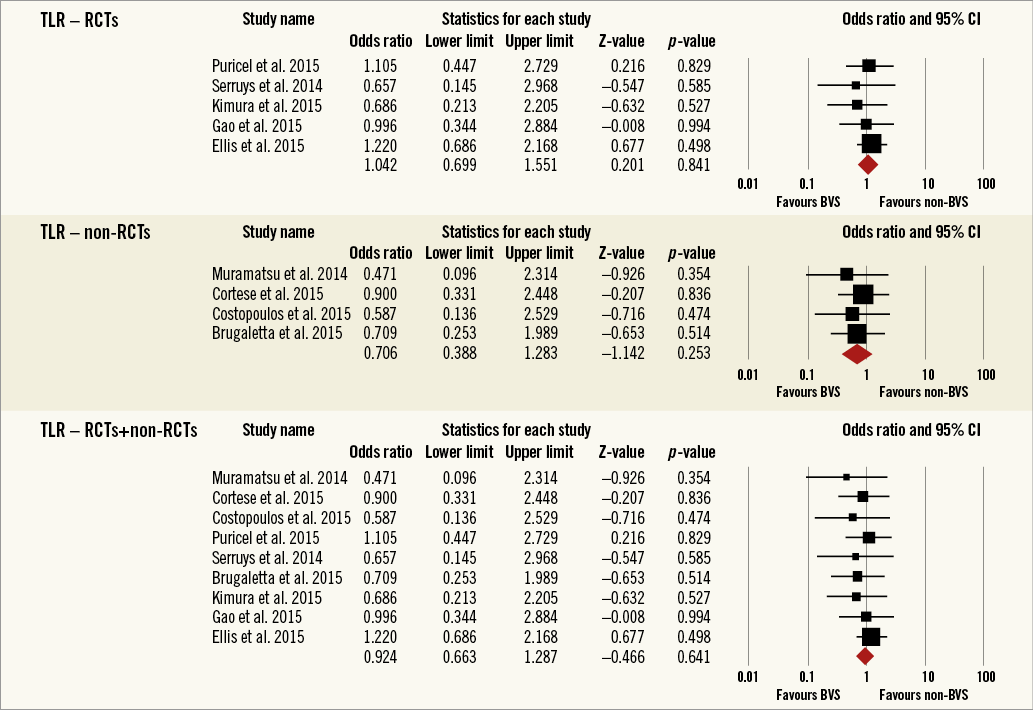
Figure 9. Forest plot displaying odds ratios (ORs) and 95% confidence intervals (CIs) for target lesion revascularisation in patients treated with bioresorbable vascular stent versus conventional stents.
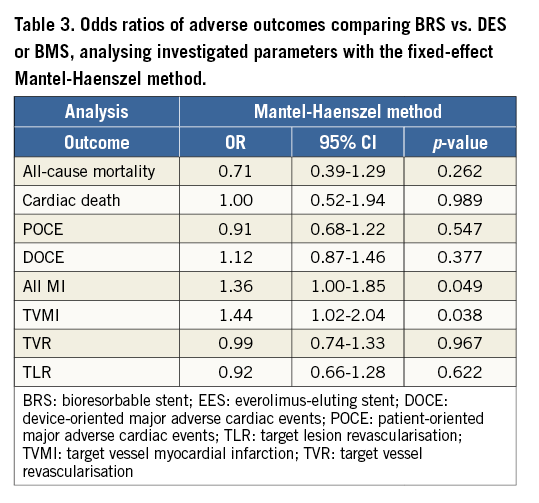
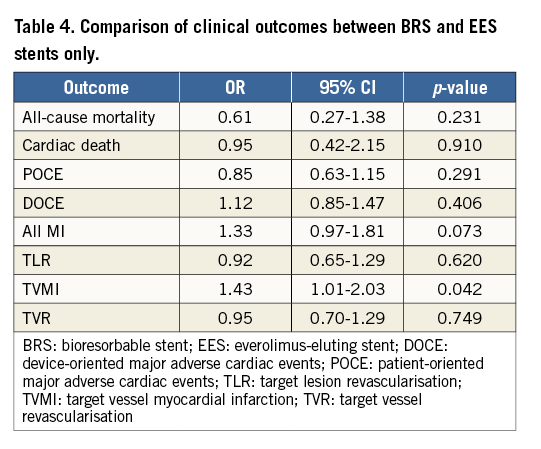
Results remained consistent when analysed with the Mantel-Haenszel method (Table 3) and when restricting the meta-analysis to the comparison of BRS vs. EES, as shown in Table 4. In the sensitivity analysis of the meta-analyses of “all-cause mortality” the effect reached statistical significance with the removal of the study by Ellis et al (2015)25. For TVMI, the pooled estimate was found to be sensitive to the studies by Cortese et al (2015), Brugaletta et al (2015), and Ellis et al (2015). None of the results remained statistically significant after applying the Bonferroni correction for multiple testing. None of the results differed significantly between randomised versus non-randomised subgroups of studies (Figure 2-Figure 9).
Discussion
To our knowledge, the current systematic review and meta-analysis is the largest analysis from available studies on the impact of BRS versus DES/BMS on all-cause mortality and cardiovascular outcomes. Our study identified a potential signal of increased risk of TVMI and all MI with BRS versus conventional stents. We observed no significant differences in the risk of all-cause mortality, cardiac death, POCE, DOCE, TVR, and TLR. These results are similar to those seen in real-world PCI registries comprising various complex lesions, where the patients treated with BRS had similar one-year clinical outcomes and procedural success compared to those treated with DES stents27. Since recent registry data indicated some signals of scaffold thrombosis after BRS implantation, a recent trial tested this supposition, but no correlation between the total surface area of the implanted BRS and the levels of on-treatment platelet reactivity was observed (r=-0.10, p=0.16)28. Our meta-analysis essentially differs from the one recently published by Cassese et al29. In order to evaluate the largest group of patients with BRS, we did not limit our literature search to RCTs, but also included prospective studies and RCT sub-analyses, and consequently we included 10 studies (with six in the Cassese et al meta-analysis29) with a total of 5,773 subjects (there were 3,738 patients in their analysis29). Our inclusion criteria also did not allow including the TROFI II study30, which was included in the Cassese et al meta-analysis29, mainly due to the fact that it was not designed to assess any clinical outcomes (and consequently the number of observed events was very low – between 0 for death and two for TLR)29,30. Finally, the authors did not evaluate an important outcome - currently widely discussed – namely TVMI, which in our meta-analysis was significantly higher for BRS in comparison to conventional stents. Also, they did not discuss the numerically higher prevalence of any-cause MI in BRS patients (5.2% vs. 3.5%) with a trend to statistical significance (p=0.06)29. These important differences mean that our meta-analysis represents the largest patient population analysed and is likely to remain the best evidence base for assessment of everolimus-eluting BRS versus conventional ones.
Of importance, BRS might provide intrinsically less kinetic support than metallic DES, especially after six to 12 months after implantation when the radial strength is diminished as a consequence of resorption kinetics31. This is one of the mechanisms that might be responsible for the numerically higher risk of any-cause MI with BRS versus DES/BMS implantation (p=0.064; and significant in Mantel-Haenszel method analysis: p=0.049), and the significant increase of TVMI noticed in this meta-analysis; however, these results still merit further investigation in trials with longer follow-up. Furthermore, BRS might entrap more thrombotic content between the vessel and the scaffold, because of the larger wall surface coverage and the greater strut thickness than the current thin-strut DES. These results can also be correlated with a greater incidence of stent thrombosis in the BRS arm reported by some studies23. Karanasos et al32 also suggested that suboptimal implantation with incomplete lesion coverage, underexpansion, and malapposition comprise the main pathomechanism for both early and late BVS thrombosis, similar to metallic stent thrombosis. It is also necessary to mention other possible technical limitations of deploying BRS, including the degradation or disintegration of stents, flaking, proinflammatory and other properties of the biomaterials31-33. It might be the result of the so-called “learning curve” but most potential triggers for BVS thrombosis could be avoided32,33. In a recently published study, Puricel et al34 investigated occurrence rates, clinical and angiographic characteristics, and possible mechanisms of scaffold thrombosis (ScT) in patients undergoing BVS implantation. The incidence rate of ScT was 1.8% at 30 days and 3.0% at 12 months. Most ScTs (52%) presented as STEMI. In multivariable analysis, ostial lesions (p=0.049) and impaired left ventricular ejection fraction (p=0.019) were independently associated with ScT. Twenty-one percent of the ScTs occurred in patients who had suspended dual antiplatelet therapy. Lower post-procedural minimum lumen and reference vessel diameters were hallmarks of ScT (p<0.0001 for all). When a BVS-specific implantation strategy was implemented, 12-month ScT rates fell from 3.3% to as low as 1.0%34.
ABSORB STEMI: the TROFI II trial is a new prospective study designed in a head-to-head manner to compare the neointimal healing score based on intraluminal filling defects, malappossed stent struts or intensive neointimal proliferation in 191 STEMI patients randomly treated with BRS versus a metallic everolimus-eluting stent30. Despite higher thrombogenic conditions, the results obtained at six months have shown almost total arterial healing and low healing scores with both types of stent implantation, and have reinforced the possibility of platelet therapy interruption before one year30. Another prospective randomised study – A Prospective, Randomized Trial of BVS Versus EES in Patients Undergoing Coronary Stenting for Myocardial Infarction (ISAR-Absorb MI) trial (NCT01942070) – is currently ongoing and the results are expected relatively soon35.
Various factors related to the patient-related background patho–logy, types of lesion, the technique of implantation (correct predilatation, expansion, and apposition), the type of scaffold (strut/polymer thickness, antiproliferative agent, drug dosage) and the experience of operators definitely influence these results36. Indeed, patient-related predisposition to increased thrombosis could be a decisional factor in this meta-analysis, since three studies included patients with acute coronary syndromes17,21,22. Moreover, the bioresorbable stent Absorb is bulkier than the drug-eluting stent XIENCE in terms of its crossing profile (1.4 mm for the Absorb scaffold vs. 1.1 mm for the XIENCE stent), with a strut thickness of 150 µm, compared with 80 µm for the XIENCE stent18.
The process of neointimal coverage and late apposition status of the BRS when implanted in the highly thrombogenic setting of STEMI were evaluated using optical coherence tomography (OCT) in 50 patients enrolled in the PRAGUE-19 study37. The authors noticed differences in incomplete strut apposition and early neointimal coverage after BRS implantation37. At one year, it has been shown that BRS is replaced by connective tissue and smooth muscle cells, overcoming the issue of metal persistence into the coronary vessel wall, and may stabilise a thin-cap fibroatheroma with a neointimal layer22. BRS are also less adaptive to intense post-dilatation because of the risk of polymeric strut fracture when expanded to larger diameters compared to DES (to maximum 0.5 mm post-dilatation in BRS)38. Therefore, an optimal lumen expansion and vessel patency are absolutely essential prior to scaffold delivery. Despite all these differences compared to other types of stent, BRS achieved a similar delivery rate and good procedural outcome with DES in all studies17-26.
A new system which combines antiproliferative myolimus with a poly-L-lactic acid-based scaffold created to offer vessel support and neointimal suppression was successfully tested in 16 patients included in the DESolve first-in-man (a non-randomised, consecutive enrolment evaluation of the DESolve myolimus-eluting bioresorbable coronary stent in the treatment of patients with de novo native coronary artery lesions) trial7. The results are promising, demonstrating no chronic recoil, low in-scaffold late lumen loss, low ratio of neointimal volume at six months, and preservation of lumen patency, no scaffold thrombosis and no major adverse cardiac events (MACE) specifically attributable to the scaffold at 12 months7.
Limitations
Our meta-analysis has important limitations. The studies included had heterogeneous characteristics of patients, different study protocols/design, and a different strategy of therapy and implantation procedures across centres. In the majority of studies the type of lesion was similar in both the BRS and DES arms, but there were type A and type B1 lesions. Only one study reported lesions as in the real world and used BRS in bifurcation, coronary chronic total occlusions with good procedural outcome and similar results to DES. Furthermore, a degree of bias might have been present in relation to the patient selection for BRS implantation, with a possible impact on these findings. Next, in most of the included studies (7/10) myocardial infarction was defined as all MI/any-cause MI without distinction between periprocedural events and late “spontaneous” MIs (only in two studies was MI defined as any periprocedural event and not during the procedure/follow-up MI, and in one as in-hospital/follow-up MI). Finally, although the difference between BRS and non-BRS stents in terms of all-cause mortality and MI did not reach statistical significance, the relatively large point estimates and wide confidence intervals suggest that our results might be inconclusive. Further investigation is merited.
Conclusion
In conclusion, our meta-analysis suggests a significantly higher risk of TVMI with BRS compared with conventional stents and a numerically higher risk of all MI and no significant differences in the rates of occurrence of the other outcomes during one-year follow-up. Further studies with larger samples sizes, longer follow-up, different clinical scenarios and more complex lesions are required to confirm or refute our findings.
| Impact on daily practice The available data, including the recently published ABSORB III and ABSORB China trials, suggest that an everolimus-eluting bioresorbable stent was non-inferior to an everolimus-eluting conventional stent for the primary endpoints at one year. Our meta-analysis suggests a significantly higher risk of TVMI with BRS compared with conventional stents and a numerically higher risk of any-cause MI. No significant differences in the rates of occurrence of the other outcomes were observed during one-year follow-up. Further studies, including the ongoing ABSORB IV trial, with a larger sample size, longer follow-up, different clinical scenarios and more complex lesions are required to confirm or refute our findings. |
Guest Editor
This paper was guest edited by Carlo Di Mario, MD, PhD, FRCP, FESC; NHLI Cardiovascular Biomedical Research Unit, Royal Brompton Hospital, London, United Kingdom.
Acknowledgements
The meta-analysis has been prepared within the Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group (www.lbpmcgroup.umed.pl).
Conflict of interest statement
The authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

