Introduction
A large body of evidence is available for biodegradable polymer stents1, but long-term data are scarce and predominantly evaluated for Caucasian subjects. The BIOFLOW-IV trial aimed to provide additional data, comparing the safety and effectiveness of the ultrathin Orsiro biodegradable polymer sirolimus-eluting stent (BP-SES; Biotronik) with the XIENCE Prime/XIENCE Xpedition durable polymer everolimus-eluting stent (DP-EES; Abbott). We herein report final 5-year data.
Methods
The BIOFLOW-IV study has been described previously2, and is registered at ClinicalTrials.gov: NCT01939249. In brief, BIOFLOW-IV is a randomised controlled (2:1), intercontinental, multicentre, non-inferiority trial. Between September 2013 and January 2015, patients were enrolled at 46 sites in Japan, Europe, Israel and Australia. Eligible patients had stenotic de novo lesions in up to 2 separate native coronary arteries. Dual antiplatelet therapy (DAPT) was recommended for at least 6 months. Follow-up was scheduled at 30 days, 6 and 12 months, and annually thereafter up to 5 years. An independent clinical events committee adjudicated events. Endpoints beyond 12 months are listed in Supplementary Table 1.
The study was performed in accordance with ISO 14155:2011, Japanese Good Clinical Practice guidelines, the Declaration of Helsinki, local and national regulations and was approved by all institutional ethics committees. All patients provided written informed consent. The study was monitored with 100% source data verification.
The Orsiro BP-SES is based on an ultrathin cobalt-chromium stent combined with a unique hybrid coating. The passive coating reduces ion release from the stent and minimises the interaction between the stent and tissue, and the active coating releases sirolimus through a biodegradable polymer matrix2.
The statistical methods are provided in the Supplementary Appendix 1.
Results
Data up to 12 months have been reported previously2. In brief, 385 patients were included in the BP-SES group and 190 patients in the DP-EES group. Core laboratory-assessed lesion lengths, reference vessel diameters and percent diameter stenosis were 13.6±6.2 mm, 2.76±0.48 mm, and 66.0±12.6 %, respectively.
At 60 months, 19.3% of patients (99/512) were on DAPT, and 8.2% (42/512) had additional antiplatelet or anticoagulation therapy. A total of 90.4% (463/512) were symptom free, and the remaining patients had predominantly stable angina.
Clinical information at 60 months was available for 94.8% in the BP-SES group and 96.3% in the DP-EES group. Outcomes were similar amongst the groups, with target vessel failure (TVF) estimates of 12.3% for the BP-SES group versus 10.8% for the DP-EES group (p=0.652) (Table 1, Figure 1).
A comparison between patients enrolled in Japan and those enrolled outside of Japan is provided in the Supplementary Appendix 2 and Supplementary Table 2–Supplementary Table 5. Figure 1 includes a comparison between BP-SES and DP-EES in the Japanese region. Patients enrolled in Japan were significantly older, had more hypertension, hypercholesterolaemia, prior stroke or transient ischaemic attack, more previous coronary interventions and were more frequently on DAPT at 60 months, while lesion parameters were similar across the regions.
Table 1. Kaplan-Meier failure estimates of clinical outcomes up to 60-month follow-up.
| 24 months 730 days |
36 months 1,095 days |
48 months 1,460 days |
60 months 1,825 days |
Log-rank p-value |
|||||
|---|---|---|---|---|---|---|---|---|---|
| BP-SES | DP-EES | BP-SES | DP-EES | BP-SES | DP-EES | BP-SES | DP-EES | ||
| TVF, universal def | 30 (7.9) | 17 (9.1) | 39 (10.4) | 18 (9.6) | 44 (11.8) | 18 (9.6) | 46 (12.3) | 20 (10.8) | 0.652 |
| TLF, universal def | 19 (5.0) | 11 (5.9) | 23 (6.1) | 12 (6.4) | 26 (6.9) | 12 (6.4) | 27 (7.2) | 14 (7.6) | 0.863 |
| Death, MI, TVR | 44 (11.5) | 21 (11.1) | 62 (16.3) | 29 (15.4) | 70 (18.5) | 32 (17.0) | 78 (20.6) | 36 (19.2) | 0.696 |
| Death, MI | 24 (6.3) | 13 (6.9) | 33 (8.7) | 20 (10.7) | 38 (10.0) | 22 (11.7) | 43 (11.4) | 26 (13.9) | 0.401 |
| Death | 11 (2.9) | 5 (2.7) | 15 (4.0) | 11 (5.9) | 19 (5.0) | 14 (7.5) | 21 (5.6) | 15 (8.0) | 0.269 |
| Cardiac death | 2 (0.5) | 1 (0.5) | 3 (0.8) | 1 (0.5) | 4 (1.1) | 1 (0.5) | 4 (1.1) | 2 (1.1) | 0.985 |
| MI, universal def | 15 (3.9) | 8 (4.3) | 21 (5.6) | 9 (4.8) | 23 (6.1) | 9 (4.8) | 26 (7.0) | 12 (6.6) | 0.856 |
| TV-MI, universal def | 14 (3.7) | 7 (3.7) | 16 (4.2) | 8 (4.3) | 17 (4.5) | 8 (4.3) | 17 (4.5) | 9 (4.9) | 0.856 |
| CD-TVR | 18 (5.9) | 11 (6.0) | 29 (7.8) | 12 (6.5) | 33 (8.9) | 12 (6.5) | 35 (9.5) | 13 (7.1) | 0.372 |
| CD-TLR | 19 (2.7) | 3 (1.6) | 25 (3.2) | 3 (1.6) | 28 (3.8) | 3 (1.6) | 15 (4.0) | 3 (1.6) | 0.137 |
| Probable or definite ST | 3 (0.8) | 0 (0.0) | 3 (0.8) | 0 (0.0) | 3 (0.8) | 0 (0.0) | 3 (0.8) | 0 (0.0) | 0.554 |
| Cerebrovascular events | 7 (1.8) | 8 (4.3) | 8 (2.1) | 8 (4.3) | 11 (3.0) | 9 (4.9) | 14 (3.8) | 11 (6.0) | 0.221 |
| Data are displayed as n (Kaplan-Meier estimate in %). BP-SES: biodegradable polymer sirolimus-eluting stent; CD-TLR: clinically driven target lesion revascularisation; CD-TVR: clinically driven target vessel revascularisation; MI: myocardial infarction; DP-EES: durable polymer everolimus-eluting stent; ST: stent thrombosis; TLF: target lesion failure; TVF: target vessel failure, TV-MI: target vessel myocardial infarction | |||||||||
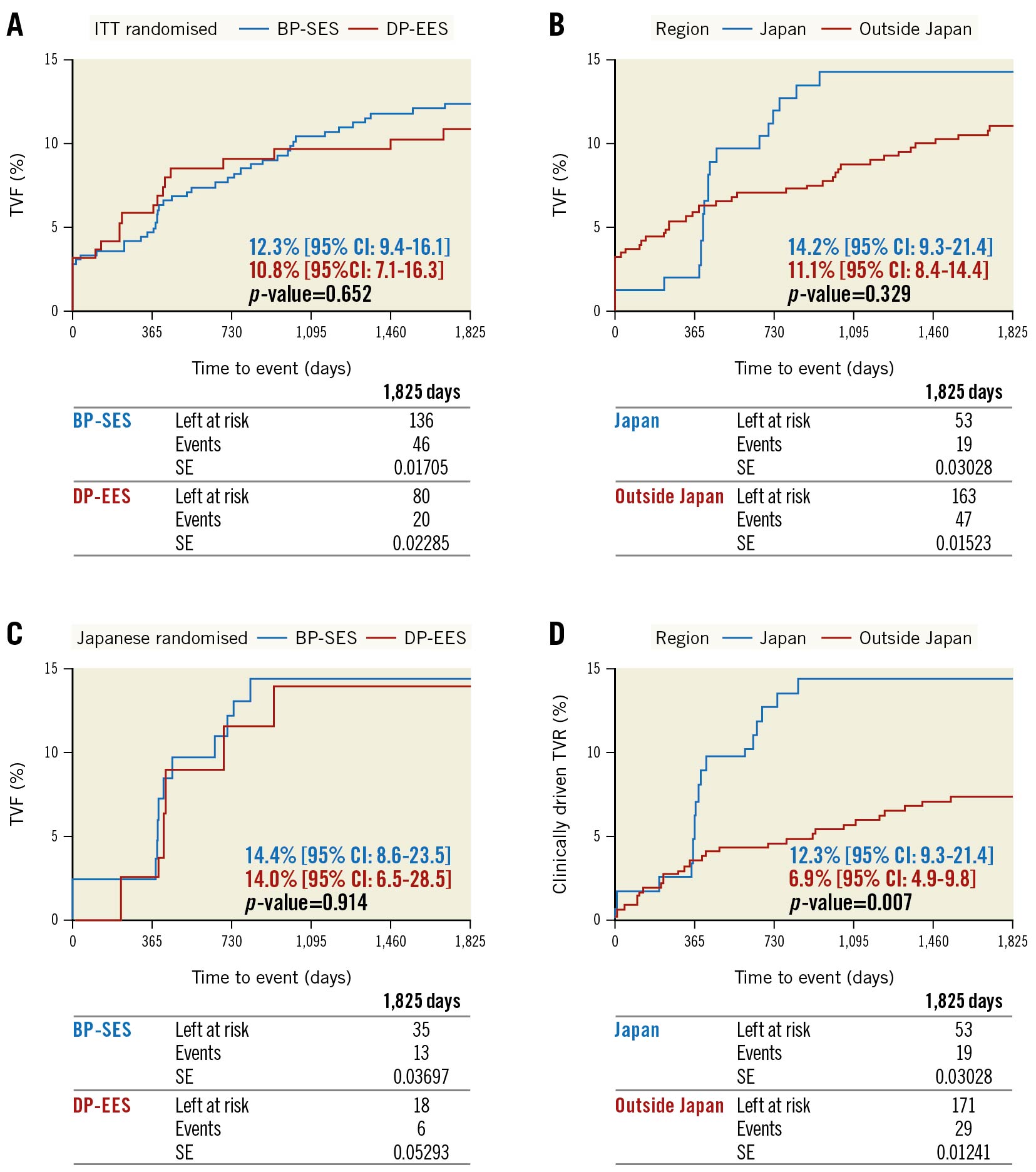
Figure 1. Kaplan-Meier estimates for target vessel failure and clinically driven target vessel revascularisation. There was no significant difference in TVF between BP-SES and DP-EES (A), but comparing Japanese with non-Japanese centres, the Japanese centres had a non-significant trend towards a higher TVF rate as the curves rise steeply around 1 and 2 years (B). However, there was no difference in TVF amongst BP-SES and DP-EES within the Japanese region (C). The difference in TVF between Japan and outside of Japan was based on a significant trend towards higher CD-TVR (D), which was the underlying cause for all TVF. A likely explanation is that – although no diagnostic repeat angiographies were foreseen according to the study protocol – many Japanese centres performed routine angiographies, whereas in centres outside Japan the follow-up visits were mainly conducted by phone. This phenomenon might be caused by a difference in local practices, but also by the fact that the Orsiro BP-SES was not market approved in Japan at the time of enrolment, which usually triggers a more thorough follow-up, particularly as the device name was replaced with a code (BTR-1131). It is well known that routine angiographic follow-up increases revascularisation rates5. Additionally, there is a steep increase in TVR depicted for the Japanese cohort around 12 and 24 months, the time of angiographic follow-up and the CD-TVR was higher in patients with routine angiographic follow-up in Japan versus those without (14.6% vs 7.0%; p=0.211) (Supplementary Table 3). BP-SES: biodegradable polymer sirolimus-eluting stent; CD-TVR: clinically driven target vessel revascularisation; CI: confidence interval; DP-EES: durable polymer everolimus-eluting stent; ITT: intention-to-treat; SE: standard error; TVF: target vessel failure
Discussion
The BIOFLOW-IV randomised controlled trial demonstrated a sustained treatment effect at 60 months with very good clinical outcomes in both treatment groups. The 5-year TVF rate was 12.3% for the BP-SES group and 10.8% for the DP-EES group and is thus consistent with similar studies using contemporary drug-eluting stents, e.g., the EVOLVE II Trial with 5-year TVF rates of 18.2% and 18.1%3 and the CENTURY II trial with TVF rates of 12.5% and 11.3%4 for biodegradable and durable polymer stents, respectively.
The rate of symptom-free patients at 5 years was high at 90%, and the stent thrombosis rate was low, consistent with previous series. An individual patient data analysis of the BIOFLOW studies, encompassing 3,717 patients, reported only 13 cases of definite or probable stent thrombosis in 2,923 patients treated with the BP-SES1. The 3 cases of definite or probable stent thrombosis reported in the BP-SES group in BIOFLOW-IV were all acute (≤24 hours) and were not necessarily related to the stent itself: one occurred in a patient with extensive dissections after predilatation that was likely not fully covered by the stent, 1 patient was treated outside the protocol as he had continued ST-elevation and elevated cardiac enzymes prior to the procedure, and 1 patient had clopidogrel resistance. No further definite or probable stent thrombosis occurred up to 5 years.
Limitations
The study was powered for non-inferiority but not for differences between Japanese and non-Japanese centres, nor was it blinded. Further, inclusion and exclusion criteria were restrictive to comply with Japanese regulatory purposes. Lastly, the use of different definitions for myocardial infarction across studies hampers the comparison of outcomes.
Conclusions
The intercontinental, randomised controlled BIOFLOW-IV study demonstrated sustained safety and performance of the BP-SES and the DP-EES at 60 months, with low event rates that were similar amongst the groups and the absence of late or very late definite or probable stent thrombosis.
Acknowledgements
The authors thank Beatrix Doerr, a medical writer, for her editorial assistance, which was reimbursed by Biotronik.
Funding
The BIOFLOW-IV study was funded by BIOTRONIK AG, Switzerland.
Conflict of interest statement
T. Slagboom declares having a personal consultancy agreement with BIOTRONIK before and during the study. R. Waksman reports grants and personal fees from Abbott Vascular, AstraZeneca, Biosensors, BIOTRONIK, Boston Scientific, and Chiesi; personal fees from Amgen, Corindus, Lifetech Medical, Medtronic, Philips/Volcano, and Pi-Cardia Ltd; is an investor in MedAlliance; and receives grants from Edwards Lifesciences. R. Toelg declares receiving speaker honoraria from BIOTRONIK. B. Witzenbichler declares having received financial support from BIOTRONIK for the study coordinator's labour costs. M. Haude reports study grants and personal fees from BIOTRONIK, Abbott Vascular, Cardiac Dimensions, and Philips. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

