The “Will this trial change my practice?” sessions at PCR
The aim of the article is to capture the session at EuroPCR 2015, communicate the analysis of the trialists, and report the views expressed in the interactive discussion.
Abbreviations
ACS: acute coronary syndrome
BMS: bare metal stent
DAPT: dual antiplatelet therapy
DES: drug-eluting stent
ESC: European Society of Cardiology
HCRI: Harvard Clinical Research Institute
LAD: left anterior descending artery
LCx: left circumflex artery
LM: left main coronary artery
MACCE: major cardiac and cerebrovascular events
MI: myocardial infarction
ST: stent thrombosis
Introduction to the session
The Dual Antiplatelet Therapy (DAPT) study, comparing 12 with 30 months of dual antiplatelet therapy after percutaneous coronary intervention (PCI) with stent implantation1,2, was the focus of the discussion during the dedicated session at EuroPCR 2015 entitled “Will this trial change my practice?”. The session was chaired by Thomas Cuisset (France) and co-chaired by Jean-Francois Tanguay (Canada), who was involved in the DAPT study as a member of the DAPT study Advisory Committee. The invited panellists were all active interventional cardiologists with extensive experience in conducting clinical research. Andreas Baumbach (UK) presented a clinical case during the session to illustrate the clinical challenge to selecting the optimal duration of DAPT. Tony Gershlick (UK), who was involved in the DAPT study as a member of the advisory committee, UK national coordinating investigator and local participating investigator, outlined what was already known from the literature before the DAPT study. Alexandra Lansky (USA) provided insightful perspectives as an experienced trialist on the methods, results and conclusion of the DAPT study. The session was rounded off by Andreas Baumbach who revealed how he had in fact treated his patient, and the session was concluded with some closing remarks and key take-home messages by Jean-Francois Tanguay.
The case presentation
A case was presented by Andreas Baumbach which clearly illustrated the clinical challenge in selecting the optimal choice and duration of DAPT. The patient was an 86-year-old male who initially presented with an acute inferior myocardial infarction (MI). The patient was known to have renal impairment. He was loaded with aspirin and clopidogrel and underwent primary PCI with DES placement in the ostium of the right coronary artery (RCA) and in the distal RCA (culprit lesion). The patient had coinciding heavily calcified lesions in the left anterior descending (LAD) artery, left circumflex (LCx) artery and the left main (LM) artery which were initially left untreated but subsequently needed intervention due to recurrence of ischaemic chest pain. After being turned down by the surgeons, the patient underwent elective PCI with rotational atherectomy and, in total, four DES in the LAD, LCx and LM with a good final angiographic result.
The audience was polled about the optimal DAPT strategy for this 86-year-old ACS patient with renal impairment who had undergone complex multivessel and left main PCI with a total of six DES. Alexandra Lansky started the discussion suggesting re-loading the patient with the more potent thienopyridine ticagrelor because of the high ischaemic risk in this patient (ACS presentation with multiple stents after complex PCI), even though the patient had a higher bleeding risk due to age and renal impairment. Chairman Thomas Cuisset asked the audience to vote and the majority indeed agreed to switch to ticagrelor. Then, he asked the audience about their current practice and how they would treat this particular patient. The majority of the audience would follow the guidelines of the European Society of Cardiology (ESC) of administering one year of DAPT after ACS3-5. Andreas Baumbach then raised the question whether anyone would prolong DAPT duration beyond one year. Thomas Cuisset noted that, except for age, the patient may benefit from a prolonged duration of DAPT because of the clinical presentation and the complex PCI including LM stenting. Tony Gershlick reminded everyone of the difficulties in balancing the risks (of bleeding) and the benefits (i.e., decreasing ischaemic events), because factors predicting long-term ischaemic events sometimes also predict bleeding events. Again, the audience voted and only one attendee would have prescribed DAPT for more than one year.
Background: what was known before the trial?
Tony Gershlick presented a clear overview of the available data before the DAPT study. He reminded us that it was 2004 when the first reports on stent thrombosis (ST) cases after the use of DES were reported. A meta-analysis presented by Camenzind during the ESC conference in 2006 suggested not only an increased risk of ST, but also an excess mortality risk with DES compared to bare metal stents (BMS). DES not only inhibited smooth muscle cell proliferation preventing intimal hyperplasia and thus in-stent restenosis, but also delayed the bystander endothelial coverage, increasing the risk of ST. ST events are associated with a high risk of mortality (mortality rate after definite ST: 35%). The general idea evolved that DAPT was necessary to prevent ST during endothelial healing. Indeed, studies in the first-generation DES era showed that premature DAPT discontinuation was a very strong independent predictor for the occurrence of ST. So it was clear that DAPT use in DES was important, but for how long?
Tony Gershlick showed that, in December 2006, the FDA laid down that DAPT should be extended to at least 12 months after PCI using DES. He also pointed out that this 12-month cut-off point for DAPT duration was arbitrary and not really supported by clinical data, since clinical registries at that time all showed a continuous annual risk of ST beyond 12 months. So why stop at 12 months? On the other hand, it has been shown that the majority of ST events occur early (<30 days) and especially so for newer-generation DES including those with biodegradable polymers. The interventional drift was to shorten DAPT duration to six or even three months. All meta-analyses performed before the DAPT study showed that there were no differences in ischaemic events but a reduction in bleeding events with shorter (i.e., three to six months) DAPT duration compared to 12 months DAPT or longer. So, prior to the DAPT study the consensus was to shorten DAPT duration to avoid bleeding events, and with the newer-generation DES this would not be at the cost of more ischaemic events. Other meta-analyses on longer duration showed that the DAPT study was the only trial showing an increased mortality risk with prolonged DAPT duration beyond 12 months (which will be discussed further below), while the other trials (“DES late” and “ARCTIC Interrupted”) did not show an excess mortality risk with prolonged DAPT.
In conclusion, the evidence prior to the DAPT trial showed that a shorter (<12 months) duration of DAPT (vs. 12 months of DAPT) did not result in significant differences in ischaemic events (including MI and ST), but reduced the risk of bleeding. An extended duration of DAPT (>12 months vs. 12 months, not including the DAPT study) showed a reduced risk of ST and MI, while bleeding risk was increased (although not statistically significant), without a significant increase in overall mortality.
Trial analysis: summary of the trialist’s critical review
Alexandra Lansky presented the published DAPT trial results and provided the audience with her critical review from a trialist’s perspective. The DAPT study, published in the New England Journal of Medicine, was a large-scale, multicentre, international, double-blind, placebo-controlled, randomised trial to evaluate the clinical benefit and risks of DAPT continuation beyond 12 months after DES or BMS implantation1,2. The study was supported by eight manufacturers (four stent companies and four pharmaceutical companies), and was designed to address a request from the US Food and Drug Administration (FDA) to evaluate the optimal duration of DAPT after DES. Patients older than 18 years, treated with FDA-approved DES or BMS and candidates for DAPT after treatment, were eligible for inclusion. Patients were first treated with one year of DAPT (open label of aspirin plus either clopidogrel or prasugrel). After one year, randomisation was performed if patients did not experience a major adverse cardiovascular event (MI, stroke, repeat revascularisation, ST) or a moderate/severe bleeding. Other exclusion criteria were patients on oral anticoagulants, a life expectancy of <3 years, or non-compliance with DAPT within the first year (compliance defined as having taken 80 to 120% of the study drug).
Patients were randomised in a 1:1 fashion to 1) continuation of the DAPT, or 2) aspirin plus placebo, up to 30 months, after which both groups were followed up until 33 months on aspirin only. The study was powered for superiority on two co-primary endpoints, ST and major adverse cardiac and cerebrovascular events (MACCE, defined as the composite of death, MI or stroke). The primary safety endpoint, powered for non-inferiority, was the incidence of moderate or severe bleeding according to the GUSTO bleeding criteria (Figure 1).
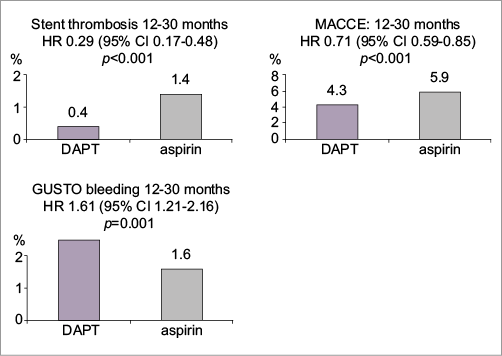
Figure 1. Clinical event rates in the two randomisation groups at month 30 (%). Stent thrombosis, MACCE (co-primary endpoints) were significantly lower with DAPT continued to 30 months compared to discontinuation at 12 months, while bleeding (primary safety endpoint) was increased with prolonged DAPT. Figure reproduced from the PCR Trials Book (© 2015 Europa Digital & Publishing).
When presenting the study results, Alexandra Lansky emphasised that only 9,961 patients were randomised at one year of follow-up (44% of enrolled patients). From the 22,866 patients treated with a DES who were initially enrolled in the trial, 56% were not randomised at one-year follow-up because of a bleeding event (n=616), an ischaemic cardiovascular complication (n=2,022), or due to withdrawal from the trial by the patient or treating physician (n=5,808), emphasising that the randomised population was in fact at low bleeding risk and lower ischaemic risk, representing a very selective PCI population. One quarter of randomised patients presented with acute coronary syndrome (ACS). Fewer than half of the patients (47%) received a contemporary DES (XIENCE everolimus-eluting stent [EES]), while now obsolete stents were used in the majority of patients, including first-generation DES use in 38% (CYPHER sirolimus-eluting stent in 11% and TAXUS paclitaxel-eluting stent in 27%) and Endeavor zotarolimus use in 13%. Finally, clopidogrel was the thienopyridine used in 65% of patients, and prasugrel was used in the remaining 35% (which was notable given that only 25% of patients presented with ACS).
Alexandra Lansky continued by presenting the clinical outcomes of the patients included in the trial who were being treated with DES - the primary analysis population of the trial. The absolute reduced risk of definite/probable ST between 12 and 30 months was 1.0% with prolonged DAPT (0.4% vs. 1.4%, p<0.001), corresponding to a number needed to treat (NNT) of 100. The other co-primary endpoint of MACCE between 12 and 30 months was also significantly reduced with prolonged DAPT (absolute difference 1.6%; 4.3% vs. 5.9%, p<0.001; NNT 63). However, this was at the cost of a 0.9% higher moderate/severe bleeding rate (primary safety endpoint) in the prolonged DAPT arm (2.5% vs. 1.6%, p<0.001, number needed to harm [NNH] 111), mostly driven by moderate bleedings (1.7% vs. 1.0%, p=0.004), while severe bleeding rates were not different between groups (0.8% vs. 0.6%, p=0.15). There was a significant overall reduction in the occurrence of MI, as well as a reduction in non-ST-related MIs when DAPT was prolonged, with 55% of MI benefit being non-ST-related but related to new patient-related ischaemic events (“secondary prevention”). However, with prolonged DAPT, there was a trend towards a higher risk of mortality at 30 months within the prolonged DAPT group (2.0% vs. 1.5%, p=0.052), which was significant at 33 months (2.3% vs. 1.8%, p=0.04). This difference was explained by an excess of non-cardiovascular deaths, which numbered 48 (1.0%) in the prolonged DAPT group and 22 (0.5%) in the 12-month DAPT group (p=0.002), with no differences in cardiovascular mortality.
Alexandra Lansky presented her thoughts on how this trial would influence our daily clinical practice. Although overall the net clinical impact of the trial is quite favourable (NNT for ST was 100, NNT for MACCE was 63, both lower than the NNH for bleeding: 111), she also clearly showed that the net clinical benefit is far less favourable with contemporary DES, with the caveat that these are subgroup analyses. For the XIENCE EES subgroup, the absolute risk reduction for ST was 0.4% (NNT 250) and for MACCE only 0.2% (NNT 500), while the absolute increase in bleeding risk was similar to other stents (1.2%; NNH 83). So, in the EES subgroup, every prevented ST will be at the cost of three moderate/severe bleedings, putting in question the clinical benefit of prolonged DAPT with current-generation DES. Another discussion point brought forward by Alexandra Lansky was the fact that the clinical benefit was largest in the ACS subgroup6. Prolonged DAPT reduced the risk of ST by 1.4% in the ACS subgroup, while this was only 0.7% in the non-ACS subgroup, and reduced MACCE by 2.9% (ACS) and 0.9% (non-ACS). The absolute differences in bleeding rates, however, were similar for ACS and non-ACS (1.1%, 1.9% vs. 0.8%, p=0.005 for ACS and 0.9%, 2.6% vs. 1.7%, p=0.007 for non-ACS)6. She calculated that the net clinical impact might balance to a net clinical benefit in the ACS subgroup: NNT was 71 for ST and 35 for MACCE, while NNH for bleeding was 91, such that more ischaemic events were prevented than bleeds caused in the ACS cohort (1.3 ST, 2.8 MI and 2.6 MACCE events prevented for every bleeding caused). However, in the non-ACS group, these numbers were 143 (NNT for ST), 111 (NNT for MACE) and 111 (NNH for bleeding), shifting the balance towards more bleeds than ischaemic events prevented. Lansky also emphasised that the ACS analysis represents all stented patients (including first-generation DES and BMS), and the benefit of prolonged DAPT among ACS patients is likely to be dampened with current-generation DES. Finally, Alexandra Lansky shared her concern with regard to the increased risk of mortality when DAPT was prolonged. A recent meta-analysis by Palmerini et al which also included the recent “ITALIC” and “ISAR SAFE” trials showed a 22% increased risk in all-cause death with prolonged DAPT7. When looking specifically at the DAPT trial data, Alexandra Lansky emphasised that the increased mortality was bleeding-related, even among the cancer-related and trauma-related deaths, therefore raising concern in systematically treating all patients with prolonged DAPT, which may best be used in select patients presenting with ACS at low bleeding risk. Her final take-home message was that the results of the DAPT study are not inconsistent with other trials seeking to demonstrate the benefits of shortened DAPT duration (i.e., three to six months), as more than half (56%) of the initially enrolled patients were excluded and not randomised at 12 months. The DAPT study excluded those very patients who might benefit from a shorter duration of DAPT at the time of PCI.
Lansky concluded that, ultimately, prolonged DAPT should be assessed in the context of contemporary DES where late ST risk is extremely low, where the benefit is mostly in secondary prevention and is associated with clear bleeding risk. In the absence of a mortality benefit and possible harm, prolonged DAPT should be used selectively among high ischaemic risk ACS patients with low bleeding risk.
Discussion between the panel and the audience
The session ended with a lively discussion between the panellists and the audience as to whether or not this trial will/should change our practice. Tony Gershlick emphasised that we should be cautious in interpreting the mortality data in DAPT because it was a secondary, underpowered endpoint, and the difference in mortality could be explained by an imbalance in baseline cancer cases or an imbalance in, for instance, the occurrence of car accidents, which have resulted in this mortality signal. Although Alexandra Lansky understood his concern, she still believed that one cannot simply dismiss the mortality findings, and more in-depth analyses are needed to look at the exact circumstances of the trauma and bleeding-related deaths. Gershlick questioned whether only to take severe bleeding into account instead of moderate and severe bleeding. Lansky responded that moderate bleeds are also related to a worse prognosis including mortality and should legitimately be included in the safety endpoint and consideration of risk benefit assessment. A member of the audience suggested accounting for patients’ risk of trauma when considering prolonged DAPT beyond one year. Andreas Baumbach reminded everyone that the original motivation of investigating prolonged DAPT was driven by stent-related complications (ST) associated with first-generation DES. While the DAPT trial confirmed the secondary prevention benefit of prolonged DAPT, as was also seen in the PEGASUS trial (ticagrelor vs. placebo in patients with a history of MI), most of the ischaemic benefit in the DAPT trial could be attributed to the reduced risk of ischaemic events in those patients treated with first-generation DES. A member of the audience noted that not only patients with a bleeding event at <1 year were excluded from randomisation in the DAPT study, but also those patients with an ischaemic event, suggesting that the benefit of DAPT might be greater if all patients with an ischaemic event at <1 year post-PCI had been included in the study. Another attendee argued that we should focus prolonged DAPT on ACS patients (as did the PEGASUS trial), who are in general at higher risk of ischaemic events. Tony Gershlick emphasised that, in contrast to PEGASUS, there was no ticagrelor used in the DAPT study.
The case resolution and the practitioner’s view
Andreas Baumbach discussed the DAPT regime he planned for his patient. His considerations were that the patient was at high risk for ischaemic events (ACS presentation), but was also at increased bleeding risk (age and renal impairment). He did not switch to ticagrelor, although in his opinion it could be considered in this particular patient. He intended to continue DAPT for 12 months according to the ESC guidelines4. If the patient did not have any ischaemic or bleeding complications at 12 months, his intention would be to stop DAPT and to continue aspirin only.
Chair Thomas Cuisset asked whether it would be different if this patient were 46 years old. Andreas Baumbach responded that, following his personal interpretation of the trial results, he would still not consider prescribing prolonged DAPT because he is not convinced that the benefit will outweigh the sustained increased risk of bleeding with contemporary DES.
Final audience poll
The chairmen polled the audience asking whether the results of the DAPT trial would change their practice. The result was that about 50% of the attendees will change their practice while the other half will not change their practice following the DAPT trial results. Most felt that, in those with high risk for ongoing and late ischaemia (e.g., ACS patients) but with low bleeding risk, prolonged DAPT beyond one year should be a serious consideration at one year if they were free from bleeding or ischaemic events.
Conclusion by the Chairperson: where do we stand now?
Jean-Francois Tanguay concluded the session by summarising that the DAPT study showed consistent results among most subgroups in decreasing the risk of ST, recurrent MI, and MACCE. However, this was at the cost of an increased risk of moderate to severe bleeding. The increased risk of late mortality remains a concern. He noted that some subgroups might benefit more than others from prolonged DAPT, while other trials have suggested that shortening of the DAPT duration to three months might be safe in selected patients. Patients with risk factors for ST were generally not included in those trials. The same applies to the DAPT study: a large number were not randomised because the patient or treating physician did not want to stop DAPT because of ST risk. His conclusions were that the DAPT study did not provide the final answer for every patient, an individualised approach is needed to decide on optimal DAPT duration, and further trials in selected groups such as the high ischaemic risk population are essential.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Session format
Chairperson: T. Cuisset
Co-chairperson: J.F. Tanguay
Participant: M. Grundeken
Introduction: the trial headlines - T. Cuisset
A case: how should I treat? - A. Baumbach
Discussion: what is common practice?
What was known before: evidence before DAPT - A. Gershlick
Trialist’s review: methods, results, conclusion - A. Lansky
Discussion and audience interaction
Case conclusion: how does this apply to my practice? A. Baumbach
Discussion and audience interaction
Session evaluation and key lessons - J.F. Tanguay

