
In the current issue of EuroIntervention, Lee and colleagues present the results of the OPTIMA-C (Optimal Duration of Clopidogrel after Implantation of Second-generation Drug-eluting Stents) trial1.
The investigators compared six months of DAPT with 12 months of DAPT after newer-generation drug-eluting stent (DES) implantation and found that the primary composite outcome of cardiac death, myocardial infarction, or ischaemia-driven target lesion revascularisation at 12 months occurred in eight of 683 patients (1.2%) in the six-month DAPT group and in four patients of 684 (0.6%) in the 12-month DAPT group (risk difference, 0.6%; 95% confidence interval [CI]: -0.4 to 1.6%), which met the predefined non-inferiority hypothesis for the trial. The safety of shorter-duration DAPT was supported by an optical coherence tomographic substudy of 60 stents in 60 patients, demonstrating favourable stent healing at six months.
The OPTIMA-C results1 are a meaningful contribution, but the findings need to be put into the context of 14 other published randomised trials2-15. While it may seem logical to pool the results of all 15 published trials1-15, a traditional meta-analysis comparing outcomes after “short” and “long” DAPT will inadvertently contain several 12-month-versus-12-month comparisons and generate a lot of statistical noise leading to faulty inferences (Figure 1). To avoid the pitfall commonly seen in traditional analyses, we created a Bayesian network16,17 to compare event rates after short DAPT of three to six months, 12 months of DAPT, and extended durations of DAPT of 18-48 months and made the following observations:
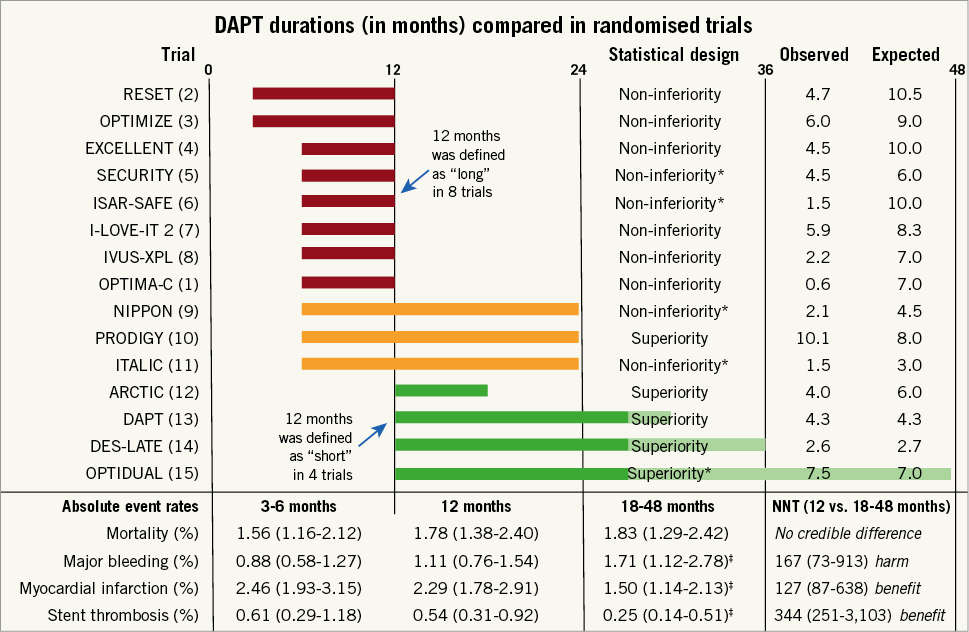
Figure 1. Trial descriptions. The upper section shows durations of dual antiplatelet therapy (DAPT), statistical design, and the observed and expected event rates for the primary endpoint for each trial. The lower section shows the absolute event rates along with 95% Bayesian credible intervals at 3-6 months, 12 months, and 18-48 months, and corresponding numbers needed to treat (NNT) per annum, derived from a network analysis and based on weighted annual event rates from a random-effects model16,17. *Trials stopped prematurely due to poor enrolment. ‡Posterior probability of superiority versus 12 months, >0.950.
– Superiority trials. Of the five trials that tested a superiority hypothesis10,12-15, three failed to show a benefit of extended DAPT over shortened treatment duration10,12,15 and one failed to demonstrate advantage of shortened over extended DAPT14. On the other hand, the DAPT trial13 was adequately powered for its endpoints, avoided a type 2 error (false negative) associated with the unrealistically large treatment effects of 40% to 50% risk reductions in the study plans of the other trials10,12,14,15, and demonstrated an advantage of extended over shortened treatment duration. We concur with the assertion18 that the DAPT trial13 was the only large, methodologically rigorous trial to date to demonstrate a robust and clinically important benefit in favour of extended treatment.
– Non-inferiority trials. Of the 10 trials that tested a non-inferiority hypothesis1-9,11, all used an absolute risk-reduction margin and claimed that short DAPT was non-inferior to extended DAPT. No trial found a bleeding advantage for shortened DAPT. All but one non-inferiority trial6 used an open-label design. Most of the non-inferiority trials had observed rates which were less than expected (Figure 1), thus widening the treatment margin and biasing the results towards non-inferiority. Power was further compromised and potential biases introduced19 in four trials that were stopped early due to slow enrolment5,6,9,11. Moreover, trials with lower than expected event rates made smaller contributions to the overall evidence. Although OPTIMA-C1 successfully met its enrolment goals, the observed rate of 0.6% for its primary endpoint was lower than the expected rate of 7% in the 12-month DAPT group. As a result, OPTIMA-C1 contributed approximately 40 times less statistical weight to the overall evidence base than the DAPT trial13 (Figure 2). As a group, the non-inferiority trials were susceptible to under-reporting of events, used heterogeneous efficacy and safety endpoints, and have generated inconclusive evidence about the optimal duration of therapy after DES implantation18.
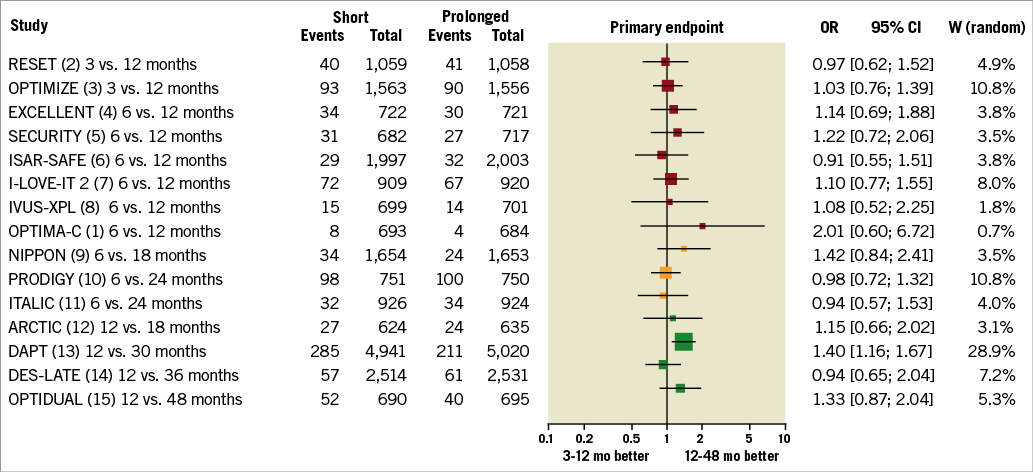
Figure 2. Trial summaries and statistical weights (W) from a random-effects model. The statistical weight of OPTIMA-C is 0.7%, but the weight of DAPT is 28.9%, or 41 times higher.
– Criticism of meta-analysis. Meta-analysis does not improve the quality of summarised evidence, cannot adjust for biases introduced by study selection or limit the risk of a type 1 error. To reduce the risk of a false positive finding, we should hold meta-analysis to the same rigorous statistical standard as a clinical trial. A prospective trial with a planned interim analysis incurs a statistical penalty, but a meta-analysis undergoing multiple iterations does not. Because we impose a higher threshold than two standard errors for a clinical trial after looking at the data during an interim analysis, we should raise the bar to declare that a finding is significant in a post hoc exercise such as a meta-analysis. Accordingly, the quality and the quantity of the meta-analytic evidence is insufficient to favour shortened DAPT as the default strategy in clinical practice.
– Mortality. An increased mortality signal observed in the DAPT trial13 and in some pooled analyses has raised concerns about using extended DAPT. However, evidentiary support for this is weak20. Mortality was not a pre-specified endpoint in any trial, and no trial provided an adequately powered comparison. Meta-analyses suggesting higher mortality with extended DAPT were associated with unadjusted p-values of 0.02 to 0.0518, which is not consistent with strong evidence. Furthermore, these reports failed to recognise that the unweighted 12-month mortality was lower in “short” trials than in the “long” trials − but should have been the same! Both a stratified meta-analysis21 and a network comparison16,17 have found no credible increase in mortality with prolonged DAPT (Figure 1). An analysis by the U.S. Food and Drug Administration has also concluded that there is no increase in overall or cancer mortality with DAPT.
– 1-6-12 and 1-3-6. The details of DAPT treatment presented in the clinical guidelines22,23 can be distilled to two simple numeric phrases. In patients at low risk of bleeding, the minimum DAPT duration is one month after bare metal stent implantation, six months after DES implantation for stable ischaemic heart disease, and 12 months after DES implantation for acute coronary syndrome. In patients at high risk of bleeding, the minimum DAPT durations are one, three, and six months, respectively22,23.
– Guidelines are not decrees. No single guideline applies to every patient in every situation. Instead, guidelines provide a framework. Because the DAPT trial13 arguably generated more insights about the usefulness of prolonged DAPT than meta-analysis, the practical conclusion is that patients who undergo newer-generation DES implantation and tolerate DAPT for at least l2 months should simply stay on DAPT.
Conflict of interest statement
The authors have no conflicts of interest to declare.

