The adoption of transcatheter aortic valve implantation (TAVI) has revolutionised the management of severe aortic stenosis (AS). Conceived as a procedure for patients who were considered high-risk or inoperable candidates for surgical aortic valve replacement (SAVR), TAVI now represents an established treatment option and is approved for all surgical risk levels. Following the results of randomised trials on TAVI in low surgical risk patients, the current guidelines no longer recommend using surgical risk alone to evaluate TAVI versus SAVR for AS treatment12. As a direct consequence, more patients will undergo TAVI at a younger age and with a longer life expectancy. This introduces two elements into the complexity of treatment choice: envisioning a lifetime management strategy and meeting patient preferences. Therefore, a pressing question arises: how does this apply to real-world clinical practice?
In this issue of EuroIntervention, Wang et al report their experience in East Denmark on the management of patients with severe AS3. They evaluated all patients referred to the Ringhospitalet Heart Team in 2021, after the most recent valvular heart guidelines were released. A unique aspect of their institution is that it captures the totality of people affected by AS from a population of 2.85 million people. All patients received cardiac computed tomography (CT), expressed their preference for TAVI or SAVR, and were subsequently evaluated in a multidisciplinary Heart Team meeting. The recommended treatment was ultimately received by 94% of the patients. From a total of 672 patients with severe AS, two-thirds (68%) were referred for TAVI. Interestingly, most TAVI recipients (77%) were at low surgical risk. SAVR was preferred in the presence of bicuspid aortic valve morphology (59% vs 16%) and ascending aorta dilation (i.e., ≥45 mm; 8% vs 2%). Nearly 40% of SAVR patients had a concomitant cardiac intervention, predominantly coronary artery bypass grafting.
The multidisciplinary Heart Team has represented the cornerstone of complex cardiovascular decision-making for over a decade. Its role is to facilitate patient-centred decision making by adopting contemporary guideline recommendations or by reaching a consensus in the absence of evidence-based indications. With the approval of TAVI for all surgical levels and its expansion to younger patients, the Heart Team must take these elements into consideration to optimise long-term patient outcomes. This study highlights the pivotal role of obtaining a cardiac CT and including patient preferences in the management of AS patients (Figure 1). At Ringhospitalet, all AS patients undergo cardiac CT analysis and express their SAVR/TAVI preference before the multidisciplinary Heart Team evaluation. Although cardiac CT is essential in assessing TAVI suitability and preprocedural planning, anticipating this exam in the decision-making algorithm might have important consequences. A CT scan can provide information about bicuspid aortic valve morphology, aortic annulus size, porcelain aorta, coronary heights, the amount and distribution of aortic annulus calcification, and peripheral vascular disease. Having this information could tip the balance in choosing TAVI or SAVR, better inform patients about procedural risks, and optimise procedural outcomes.
With TAVI being approved for all surgical risk levels, it is important to obtain and consider patient preference in the Heart Team assessment. Patients should not passively receive the Heart Team verdict, but rather be involved in the shared decision making. Every patient should be informed about their risks, expected outcomes, and implications of both TAVI and SAVR. Although the benefit/risk ratio is in favour of TAVI for the vast majority of high-risk or older patients, at lower surgical risk levels and younger age both transcatheter and surgical intervention might be reasonable. In this setting, informed patient preference is a key component to be integrated into a modern Heart Team assessment.
In 2021, most TAVI recipients (77%) in East Denmark were at low surgical risk, with a significant percentage being of a young age (38% <75 years; 18% <65 years) (Figure 1). Similar proportions have been reported in France (11.1% <65 years) and were even more pronounced in the United States (47.5% <65 years). TAVI utilisation is exponentially growing in young patients, but important knowledge gaps still exist and should be acknowledged. The NOTION trial has shown that both self-expanding TAV and SAV have good durability up to 10 years of follow-up4. However, the mean age in the NOTION trial was 79.1 years and it is unknown if such results might be valid in a younger population. Also, differences in haemodynamic function based on device design might be associated with different rates of valve degeneration at long-term follow-up5. Performing TAVI on a young person might have important implications on future interventions. Irrespective of valve durability, a young patient will likely receive one or multiple reinterventions in the decades following the index procedure. The most dreadful complication in redo-TAVI is represented by coronary obstruction6. Leaflet modification and bailout strategies (i.e., chimney stenting) might be helpful in reducing such a risk. However, it is fundamental to perform the first TAVI with a lifetime strategy plan. A young patient undergoing TAVI should ideally receive a valve with a low coronary risk plane (i.e., a good trade-off between leaflet extension and implantation depth), large upper cells, commissural alignment, and optimal valve haemodynamics.
Despite SAVR procedures being less frequent, this treatment still represents the gold standard for patients with bicuspid aortic valve anatomy. At Ringhospitalet, nearly 60% of all SAVR was performed for bicuspid AS. Bicuspid AS was a common exclusion criterion in the pivotal TAVI trials and all the evidence on TAVI safety and efficacy derives from observational registries. Most of these studies reported reassuring results for TAVI in bicuspid AS, acknowledging a higher risk of annular rupture, significant paravalvular leak, ischaemic stroke, and permanent pacemaker implantation7. Of note, these studies might be biased by the inclusion of a selected group of patients with favourable bicuspid anatomy for TAVI. Different raphe configurations and the burden of aortic valve calcification represent a real challenge for TAVI procedures, potentially resulting in suboptimal valve expansion. However, it is not known how this impacts transcatheter valve function in long-term follow-up, with available data on bicuspid AS being limited to up to 2 years after TAVI. More evidence is needed to identify the optimal valve design, refine prosthesis sizing, and explore long-term outcomes after TAVI in this setting. A dedicated randomised trial investigating the safety, effectiveness, and limitations of TAVI versus SAVR in bicuspid anatomies would help guide decision making7. Until more evidence is generated, cardiac surgery, which is agnostic to aortic valve anatomy, should be considered the preferred treatment for bicuspid AS in young and low surgical risk patients.
In conclusion, aortic stenosis management has significantly changed in the last decades. In line with increasing evidence supporting its use, TAVI is now approved for all surgical risk levels and performed in younger patients. Performing an early CT scan, obtaining informed patient preference, and envisioning a lifetime management strategy for young patients are key in the TAVI/SAVR decision process. A modern Heart Team approach should follow this algorithm to deliver individualised care plans and optimise AS treatment outcomes.
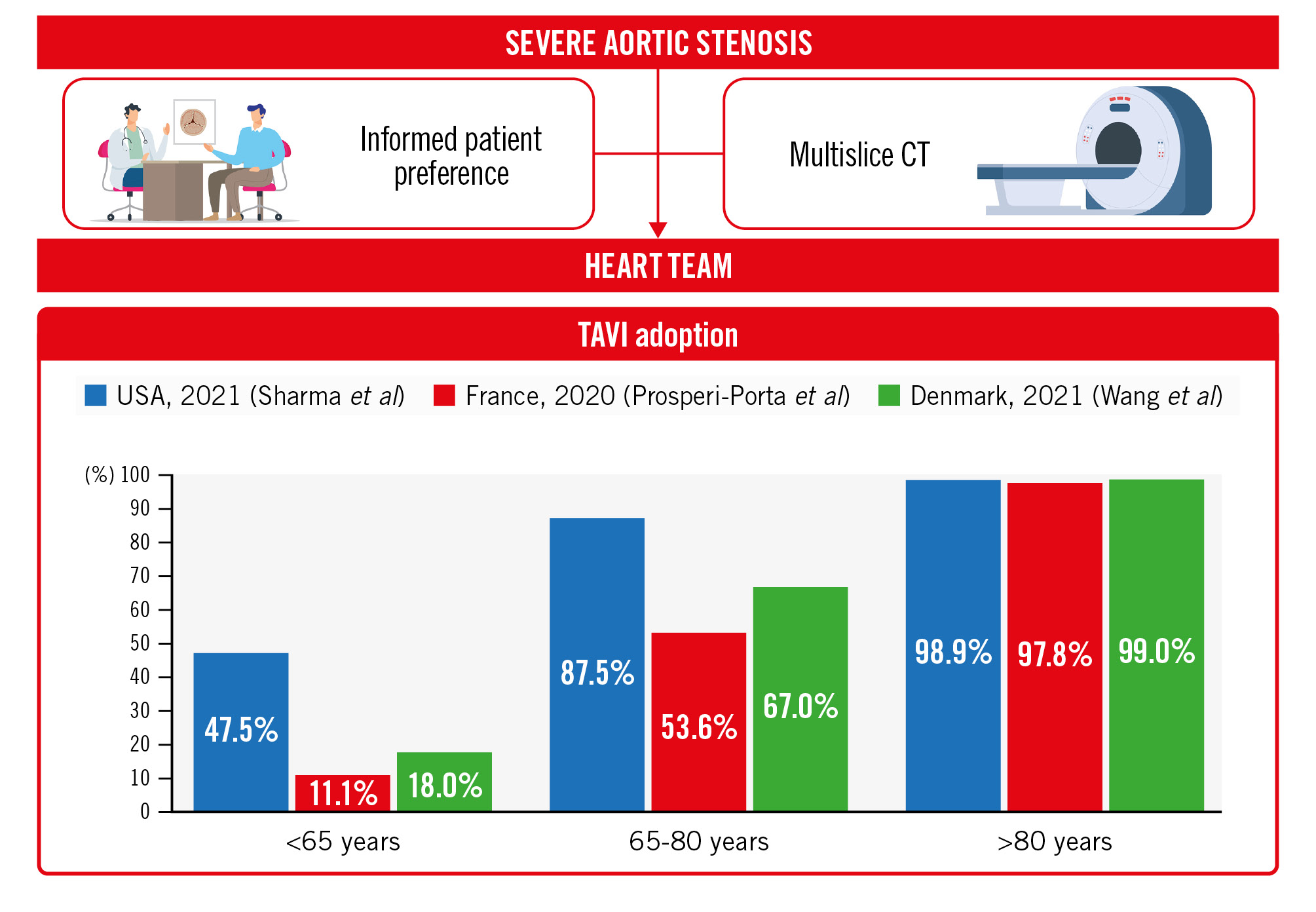
Figure 1. The modern Heart Team approach and contemporary TAVI adoption rates. CT: computed tomography; TAVI: transcatheter aortic valve implantation
Conflict of interest statement
A. Scotti has served as a consultant for NeoChord Inc. and Edwards Lifesciences. A. Latib has served on advisory boards or as a consultant for Medtronic, Abbott, Boston Scientific, Edwards Lifesciences, Shifamed, NeoChord Inc., VDyne, and Philips.

