Abstract
Aims: Primary percutaneous coronary intervention (PPCI) is the preferred strategy for acute ST-segment elevation myocardial infarction (STEMI), with evidence of improved clinical outcomes compared to fibrinolytic therapy. However, there is no consensus on how best to manage multivessel coronary disease detected at the time of PPCI, with little robust data on best management of angiographically significant stenoses detected in non-infarct-related (N-IRA) coronary arteries. CVLPRIT will determine the optimal management of N-IRA lesions detected during PPCI.
Methods and results: CVLPRIT (Complete Versus culprit-Lesion only PRimary PCI Trial) is an open-label, prospective, randomised, multicentre trial. STEMI patients undergo verbal “assent” on presentation. Patients are included when angiographic MVD has been detected, and randomised to culprit (IRA)-only PCI (n=150) or in-patient complete multivessel PCI (n=150). Cumulative major adverse cardiac events (MACE) - all-cause mortality, recurrent MI, heart failure, need for revascularisation (PCI or CABG) will be recorded at 12 months. Secondary endpoints include safety endpoints of confirmed ischaemic stroke, intracranial haemorrhage, major non-intracranial bleeding, and repair of vascular complications. A cardiac magnetic resonance (CMR) substudy will provide mechanistic data on infarct size, myocardial salvage index and microvascular obstruction. A cost efficacy analysis will be undertaken.
Conclusions: The management of multivessel coronary artery disease in the setting of PPCI for STEMI, including the timing of when to perform non-culprit-artery revascularisation if undertaken, remains unresolved. CVLPRIT will yield mechanistic insights into the myocardial consequence of N-IRA intervention undertaken during the peri-infarct period.
Introduction
Primary percutaneous coronary intervention (PPCI) is the preferred strategy for revascularisation in those presenting with ST-elevation myocardial infarction (STEMI)1. Published data indicate that up to 40% of all STEMI patients undergoing PPCI have additional significant multivessel disease (MVD) (epicardial coronary stenosis >70% in non-infarct-related arteries [N-IRAs])2-5. Whether to treat a N-IRA lesion and, if so, when can be a difficult decision. Recent attention has focused on the diffuse inflammatory nature of acute coronary syndromes, including STEMI, with multiple unstable atheromatous lesions being demonstrable in addition to the culprit one6. Thus, it may be hypothesised that multivessel PCI procedure in the peri-infarct period may reduce subsequent post PPCI adverse events by preventing the incidence of recurrent ischaemia derived from the non-infarct-related lesions. This in turn could reduce overall ischaemic burden and obviate the need for recurrent procedures. There is a suggestion both from the SWISSI II randomised trial7 and from non-randomised data8,9 that clinical outcome in stable coronary disease is related to the extent of ischaemia, and that ischaemia reduction may consequently improve outcomes. It is unknown whether complete revascularisation at the time of STEMI is beneficial. On the other hand there is certainly increasing interest in the role of microvascular obstruction as a marker of myocardial jeopardy, which conceivably may be the consequence of PCI to the N-IRA.
Currently there is no consensus on the optimal management of significant non-culprit coronary disease identified at PPCI and, in fact, there is divergent clinical practice due to a lack of a robust evidence base. Current options are: 1) to treat all significant lesions during the PPCI procedure; 2) to treat only the IRA during PPCI with further PCI to the N-IRA during same admission; 3) to perform IRA PCI only, and then further PCI based on the results of non-invasive ischaemia imaging during in-patient stay; 4) to perform IRA only, and further PCI only for recurrent ischaemic symptoms despite optimal medical therapy (OMT); 5) to perform IRA only, and determine the need for subsequent intervention on planned six to eight-week non-invasive scan looking for objective evidence of ischaemia. Recent editorials on the subject support the concept that data from appropriately powered robust clinical trials, of which there are currently none, are required to guide practice10, while noting that current guidelines advise undertaking PCI of the infarct-related coronary artery only in the absence of recurrent ventricular arrhythmia or cardiogenic shock11.
The currently available data on the management of MVD in the setting of PPCI are limited by being retrospective analyses or because they are studies conducted in a single centre, with small numbers, and which have yielded conflicting results3,5,12-22. Specifically, among retrospective studies, the US National Cardiovascular Data Registry (28,936 STEMI patients) suggests a trend towards increased mortality with multivessel PCI strategy in STEMI patients, but it is notable that it included shock patients in the analysis23. Furthermore, a recent meta-analysis by Sethi et al24 of over 32,000 registry patients also found major adverse cardiovascular events and long-term outcomes to be similar with either strategy.
By contrast, recently published data from the New York PCI registry examined both in-hospital and nine-month mortality among patients with multivessel coronary artery disease undergoing PPCI for STEMI from January 2003 to June 20063. Patients were propensity-matched and subdivided into those undergoing culprit-only PPCI (n=3,521, 87.5% of those with MVD); staged in-patient multivessel PCI (MV-PCI); or MV-PCI within 60 days. Among haemodynamically stable patients, culprit vessel PCI only during the index procedure was associated with lower in-hospital mortality than MV-PCI during the index procedure (0.9% vs. 2.4%, p=0.04). Patients undergoing staged MV-PCI within 60 days after the index procedure had a significantly lower 12-month mortality rate than patients undergoing culprit vessel PCI only (1.3% vs. 3.3%, p=0.04). The authors concluded that their findings supported the American College of Cardiology/American Heart Association (ACC/AHA) recommendation that culprit vessel PCI be used for STEMI patients with multivessel disease at the time of the index PCI provided patients are not haemodynamically compromised. Based primarily on the New York registry and on subgroup analysis of the APEX-AMI trial3,5, the ACC/AHA position recommends culprit-only PPCI in patients with MVD.
The three available randomised studies examining clinical outcomes are limited by small sample sizes, and/or short-term surrogate endpoints13,14,17. The largest and most recent of the three randomised trials of a complete versus culprit-only revascularisation strategy included 214 patients from a single centre, randomised three ways17. There was a significant reduction in major adverse cardiovascular events (MACE) during a mean follow-up of 2.5 years in the patients treated with complete revascularisation (staged or complete during PPCI) compared to culprit-only revascularisation. The endpoint was driven mainly by an increase in subsequent revascularisation although there was a non-significant trend towards increased mortality in the culprit-only arm. Other than unequal randomisation and demographic differences between groups, the major limitation was a lack of specific imaging assessment of infarct size. The need for a robust randomised comparison of conservative versus “active” management of bystander coronary artery disease following STEMI remains an important goal when one considers the evidence from the DANAMI trial25 that recurrence of angina and myocardial infarction may be reduced following appropriate revascularisation by PCI when inducible (even silent) ischaemia is detected post MI.
Table 1 indicates there is a disparity of outcome among studies, the majority of which show equivalence in outcomes between complete and culprit-only revascularisation.
Rationale and design for CVLPRIT
Given the divergence of current clinical practice, the current (CVLPRIT) trial sets out to compare outcomes from a strategy of complete in-hospital revascularisation in patients with multivessel coronary disease at PPCI, with a strategy of culprit-only PCI with subsequent revascularisation based on recurrent (ischaemia proven) symptoms. With so many treatment options in clinical practice, it is difficult to encompass each in one trial. CVLPRIT will randomise patients to one of two management strategies mirroring the broad options in current practice.
Little is understood regarding the implications of treating “bystander” lesions identified during PPCI. CVLPRIT-CMR will be the first systematic quantitative assessment of the impact of additional non-culprit-artery-related infarction and its significance from potential “additional infarction”/myocardial micro-damage, the determination of which cannot be quantified by traditional biomarkers. CMR will also be used (i) in the early post PPCI period to determine infarct size, ventricular function and volumes, and then (ii) later at follow-up to determine the extent of myocardial salvage and ischaemia26.
Methods
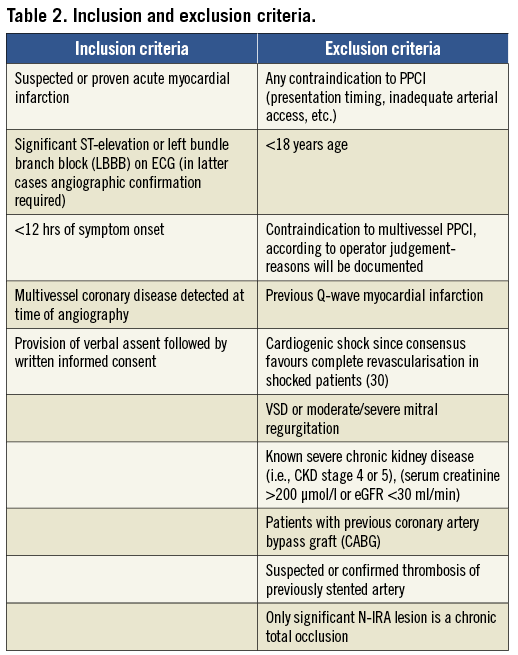
The trial is designed to enrol patients presenting for PPCI in whom angiography demonstrates multivessel coronary disease, defined as at least one lesion in a non-infarct-related artery (N-IRA) deemed angiographically significant (≥70% luminal diameter narrowing in single projection). The trial is an open-label, prospective, randomised, parallel, comparative, multicentre trial. The inclusion and exclusion criteria are set out in Table 2. Patients are enrolled into the study by a process of “assent” at the point of first medical contact (approved through the National UK Ethics Board). If significant multivessel disease is present, patients are randomised via interactive voice response system (IVRS) with stratification (see below) to: A) in-hospital complete revascularisation (preferably undertaken at the time of PPCI); or B) culprit-only revascularisation of the infarct-related artery. Subsequent revascularisation in the culprit-only arm will be driven by recurrent ischaemic symptoms during 12-month follow-up, confirmed by evidence of ischaemia on (any standard) non-invasive testing at the time of representation. Patients with multivessel disease deemed not to be suitable for randomisation will enter a documented parallel registry. All patients will undergo a planned stress nuclear myocardial perfusion scan (MPS) at 6 weeks (±2 weeks) post discharge. The results of the MPS will be “nested” (kept blind until the end of the trial), unless >20% ischaemic burden in N-IRA territory is demonstrated by the core lab, in which case the operator will be advised. There are data suggesting increasing risk consequent on high residual ischaemic burden but further intervention will be at the operator’s discretion as there is no definitive evidence of benefit25. Nesting the MPS will inform at trial publication whether routine non-invasive scan at 6 weeks (±2 weeks) follow-up actually predicts outcome and would therefore be of value in routine clinical practice.
Recurrent symptoms of angina during follow-up will be confirmed with any non-invasive ischaemia test (ETT, MPS, DSE, stress CMR) prior to proceeding to angiography and N-IRA PCI.
Primary PCI will be delivered according to local standards and in compliance with UK National and European guidelines. Antiplatelet therapy will be according to current guidelines. In those over 75 years or body weight <60 kg, a loading dose of clopidogrel 600 mg will be given followed by clopidogrel 75 mg daily in addition to aspirin. All lesions (IRA, and N-IRA if randomised) will be treated with DES unless there are concerns regarding high risk of complications with dual antiplatelet medication.
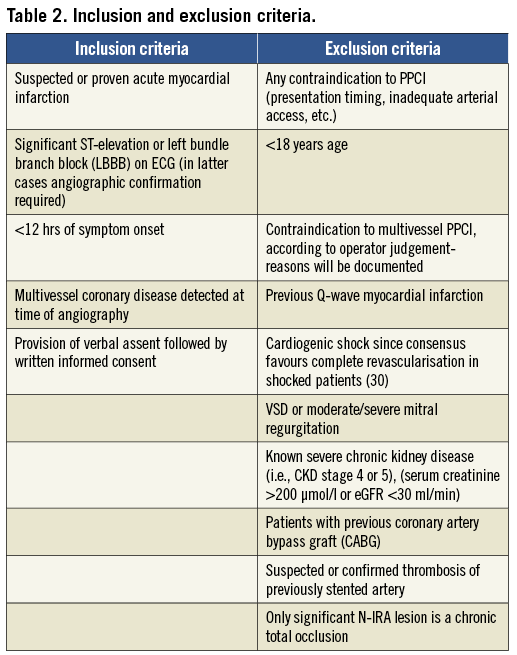
The study endpoints are listed in Table 3.
The UK National Research Ethics Service and the Clinical Trials Register have approved the study. Registration number: ISRCTN 70913605.
Statistical methods
All statistical tests are of an exploratory nature and will be completed by presenting confidence limits and descriptive p-values. The primary analysis is an intention-to-treat of all randomised patients according to treatment group. Single and composite endpoints will be expressed with 95% confidence limits and between-group comparisons done by presenting confidence limits of the corresponding difference and/or odds ratio for each clinical endpoint. While this is an exploratory study we have devised sample sizes based on available evidence published to date. The initial sample size of 144 patients per group is calculated from the accuracy of the estimation of the 95% confidence limits for each endpoint within the two treatment groups as well as for between-group comparisons. The sample size calculation was based on a primary efficacy endpoint of cumulative major adverse cardiac events (MACE). A pooled analysis of recent data from the four available randomised trials (Politi et al17, Ochala et al16, Khattab et al27, Di Mario et al14) and the non-randomised study by Qaraqani et al21 suggest the incidence of MACE in patients undergoing culprit-only revascularisation is on average around 37% at between 12 and 25 months follow-up, compared to 17-22% in patients undergoing simultaneous complete or staged (early post-discharge) complete revascularisation. Based on these figures (37% MACE in the culprit-only PCI group and 22% in the complete revascularisation group) and aiming for α 0.05 with 90% power the sample size required is 144 patients per group. We have conservatively selected a sample size of 300 (150 patients per group). Those not entering the randomised study will be followed in the registry. An adaptive design will be employed, such that review of the blinded interim clinical results will be undertaken by the independent Data and Safety Monitoring Board (DSMB) to determine whether recruitment of more than 270 patients provides sufficient statistical power based on one-year clinical outcomes and whether recruitment should be extended.
Study procedures
Patients will be recruited at seven major UK interventional cardiac centres providing 24/7 PPCI: University Hospitals of Leicester, Leicester; Wessex Cardiac Centre, Southampton; Leeds General Infirmary; Harefield Hospital/ Royal Brompton, Middlesex; Royal Derby Hospital; Kettering General Hopsital; and Royal Bournemouth Hospital.
Randomised trial
CONSENT
Patients will be asked to give “assent” to the study after being read a shortened consent form before angiography. All patients will then be asked to confirm their continued participation in the study (including the CMR scans) within 24 hours after providing full “patient information sheets” (all consent procedures have been reviewed and passed by a UK regulatory ethics committee).
RANDOMISATION
If angiographic inclusion criteria (at least one lesion in a N-IRA deemed angiographically significant [>70% luminal diameter narrowing in single radiographic projection] with the N-IRA being a major epicardial coronary artery or major branch [>2 mm]) are satisfied and there are no exclusions (all lesions should be suitable for stent implantation), the patient is randomised on-table, prior to PPCI, via dedicated 24/7 telephone randomisation service to:
– Group 1 - complete (“multivessel”) in-hospital revascularisation; or
– Group 2 - incomplete (“culprit-only”) in-hospital revascularisation.
Randomisation will be stratified by anterior or non-anterior STEMI and by the sex and age of the patient.
Registry
A register of all patients undergoing PPCI at participating centres will be completed. On admission, all patients will be asked to provide a verbal informed “assent” to participate in the research protocol study which will include the registry and RCT, and this will be documented in the patients’ hospital records. Subsequent written consent will be obtained with follow-up at one year to five years using centralised registers for death and hospital admissions. A second registry of those with MVD but deemed not suitable for randomisation with documented reason will also be kept.
Stress-rest myocardial perfusion scintigraphy will be performed in all patients 6 weeks (±2 weeks) post discharge. A technetium-99m-labelled radiopharmaceutical will be used, with adenosine stress and gated SPECT acquisitions. Clinicians will be blinded to the findings, unless the patient represents with chest pain within four weeks of the MPS and the investigation would otherwise need to be repeated on clinical grounds. This should minimise the influence of the findings on subsequent patient management and allow the unbiased predictive value of MPS on outcome to be assessed.
The flowchart for the final CVLPRIT trial protocol is outlined in Figure 1.
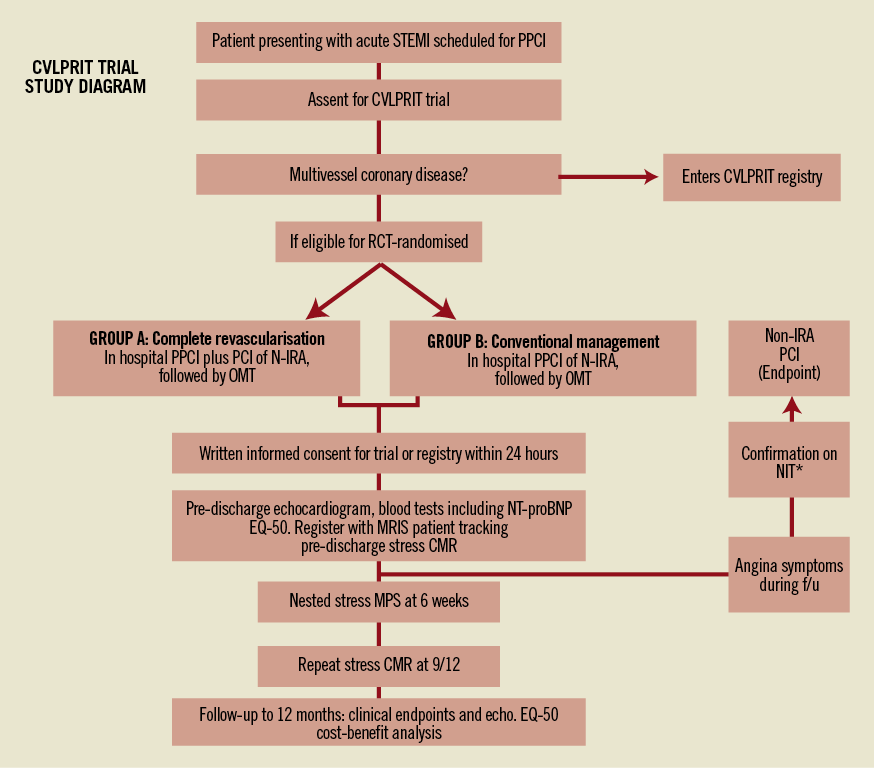
Figure 1. CVLPRIT flowchart. STEMI: ST-segment elevation myocardial infarction; PPCI: primary percutaneous coronary intervention; RCT: randomised controlled trial; OMT: optimal medical therapy; NIT*: non-invasive ischaemia testing (i.e., any form of non-invasive ischaemia test: ETT, DSE, MPS, etc.); EQ-5D: quality of life questionnaire; CMR: cardiac magnetic resonance imaging; MRIS: medical research information service; MPS: myocardial perfusion scan (nuclear scintigraphy). This result is nested and result may only be un-blinded for confirmation of ischaemic symptoms during follow-up if symptoms occur within four weeks of scan.
CVLPRIT-CMR substudy
Cardiac magnetic resonance imaging will be performed in the first 200 patients recruited who do not have contraindications to CMR. Scanning will be performed as close to discharge as possible and after additional PCI in the “complete” group.
CMR is the gold standard technique for the quantification of LV volumes and function, and delayed contrast imaging can accurately detect and quantify myocardial infarction28. Microvascular obstruction (MVO) is associated with larger infarcts and independently predicts adverse remodelling and prognosis29,30. CMR also allows quantification of myocardial salvage index which preliminary data suggest may have additional prognostic value31.
The CMR substudy will have 81% power to detect a 4% difference in infarct size in the two groups, assuming that infarct size will be 20±10% of LV mass in the culprit-only arm, alpha=0.05.
The quantification of infarct size using resting MPS with SPECT has been well validated, and has been used as a surrogate endpoint in a number of studies of adjuvant treatment in patients receiving either thrombolysis or primary PCI32. Moreover, the extent and severity of stress-induced ischaemia (strictly hypoperfusion) on MPS is widely believed to provide prognostic information in both stable patients with suspected or known coronary disease, and in those who have recently suffered an acute coronary syndrome33,34. The COURAGE trial nuclear substudy suggests that in patients with stable coronary disease randomised to PCI rather than medical management there are greater reductions in ischaemic burden, and that those patients with the most significant ischaemia reduction had fewer events during follow-up35. In the CVLPRIT study, an angiographically well-characterised group of patients will be followed prospectively post primary PCI (with or without PCI to bystander lesions). Whilst all will undergo MPS six weeks post MI, the results will generally not be allowed to influence patient management. Thus, within the limitations of its statistical power, CVLPRIT will determine: 1) SPECT infarct size in the two treatment groups as a secondary endpoint; 2) the burden of inducible ischaemia in the two groups; and 3) the prognostic significance of the ischaemic burden at 12 months relative to the assigned treatment.
Trial coordination
A central coordinating centre has been established (University Hospitals of Leicester) and it will liaise with the principal investigators. There is an independent Clinical Trials & Evaluation Unit (CTEU) responsible for overall trial management, production and entering of data into the case record forms, delivery of manual of operations, arranging meetings, data handling, quality assurance and statistical reporting, regular progress reports and newsletters being provided to all relevant parties based at the Royal Brompton Hospital. The study will be overseen by a trial steering committee consisting of the principal investigators, three independent members, one of whom will be the chairman, and a lay representative.
Additionally an independent Data and Safety Monitoring Board (DSMB) will review the clinical outcome data and an interim analysis will be performed when 120 patients have completed one- month follow-up.
Discussion
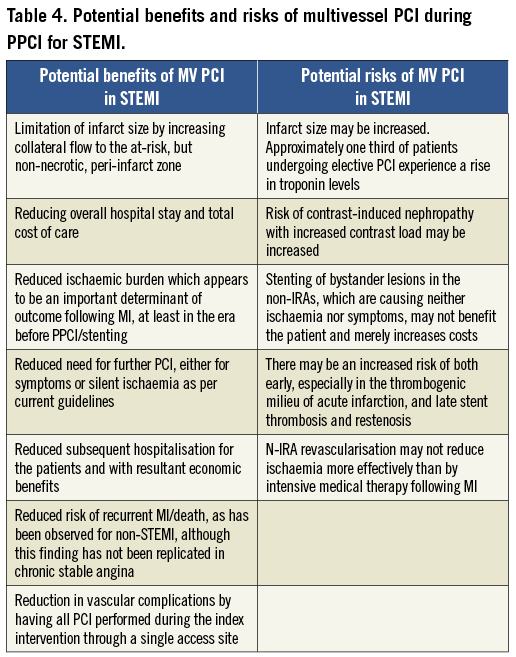
Whether multivessel coronary intervention should be attempted during the index STEMI admission is especially pertinent, not only because there is disparity among published studies as to benefit but also because conceptually it is possible to envisage the potential both for benefit and for harm by any such approach (Table 4).
Endpoint analysis
We recognise that a component of the MACE endpoint is the inclusion of need for further revascularisation and that this endpoint is more likely in the culprit-only arm. However, the more extensive revascularisation in the complete arm renders these patients at elevated risk of stent thrombosis and restenosis. Thus it would appear to disadvantage the culprit-only group although of course there may be more restenoses in the non-infarct-related arm of the complete group. However, all endpoints will be reported openly in this exploratory study as outlined in the statistical methods. The absolute clinical need to undertake unplanned interim PCI may be reasonably considered to be a MACE event, and some interim PCIs will present as new acute (chest pain events±troponin changes). The timing of the non-culprit lesion intervention may make a difference to overall outcome: non-culprit lesions treated at the time of the PPCI may induce additional ischaemia/infarction, whereas this may be less prevalent with interim non-infarct-related PCIs. Our CMR will be critical in determining what the “myocardial cost” is when non-infarct-related PCI is undertaken at the two time points, i.e., in-patient in association with the IRA PCI, or as interim. There are enough questions regarding the need for repeat PCI for us to include it as part of the primary endpoint of MACE, as have other studies such as RITA 336.
Results from the CVLPRIT study will provide data on the optimal management of multivessel coronary disease in the setting of primary PCI for acute STEMI. It will incorporate important demographic and clinical outcome data in consecutive patients not eligible for randomisation who enter the CVLPRIT registry. The nested MPS will address the utility of this test as a prognostic marker and determine whether the routine planned isotope scans that are undertaken in many centres six to eight weeks post PPCI are valid. The CMR substudy will provide important mechanistic data and will inform as to which measures of the success of revascularisation most closely correlate with outcomes. Specifically, the issue of whether to intervene early on significant “bystander” stenosis detected at the time of PPCI for STEMI remains unresolved, with a wide disparity in current clinical practice. The potential benefits for both patient and healthcare system of preventing unnecessary procedures or conversely avoiding the morbidity of recurrent ischaemia are great. CVLPRIT will illuminate and inform an increasingly important area of contemporary cardiology practice.
Acknowledgements
The main CVLPRIT study is funded by the British Heart Foundation and the CMR substudy by the National Institute of Health Research (NIHR) project grant EME_10/27/01. The NIHR comprehensive local research networks provide additional support for the MPS. Prof. Gershlick and Dr McCann additionally receive support from the NIHR Leicester Cardiovascular Biomedical Research Unit.
Conflict of interest statement
A. Gershlick is on the advisory board/speakers panel of Abbott, Boston Scientific, Medtronic Corp., Daiichi Sankyo, The Medicines Company, Astra Zenica and has trial involvement links with Boehringer-Ingelheim. N. Curzen has received grants from St. Jude Medical, Medtronic, and Haemonetics, as well as speaker/consultancy fees from St. Jude Medical, Medtronic, Boston Scientific, Haemonetics, Abbott Vascular, Lilly/Daiichi Sankyo. The other authors have no conflicts of interest to declare.

