Abstract
As transcatheter mitral replacement technologies have recently been applied in early clinical trials, the question as to whether valve implantation would have the potential to become the leading percutaneous mitral valve therapy has been raised. The aim of this report is to give an overview of the different peculiarities of percutaneous replacement and repair techniques, and to predict whether the two approaches will have a complementary rather than a competitive clinical role in the near future.
Introduction
Who will bother to perform elaborate percutaneous repair procedures, if easy-to-use percutaneous mitral valves with reproducible results become available? Will anyone care about the underlying pathology of severe mitral regurgitation or stenosis and select percutaneous repair techniques accordingly, when there is an option to “just put in a prosthetic valve”?
When looking at the surgical experience over the last decades the answers are clear: YES, physicians have to bother about elaborate percutaneous repair procedures, and yes, they have to select them wisely. The widespread use of repair procedures will, however, depend on the ability of engineers to develop devices and products that allow reproducible results with standardised procedural steps.
Percutaneous repair and replacement should and will be complementary!
Many surgical procedures have served as an inspiration for different percutaneous devices to treat mitral regurgitation (MR) in high-risk or inoperable patients over the last few years.
After more than 40,000 patients treated, transcatheter repair with the MitraClip® system (Abbott Vascular, Santa Clara, CA, USA) is the most advanced and widespread technology available for clinical use, with proven safety, efficacy and durability in different clinical settings1-3.
Different repair technologies with diverse approaches are quickly gaining popularity and have already become clinical reality, with proven feasibility and safety in their initial human investigations, including reliable annuloplasty techniques and chordal replacement4,5; many other technologies are under preclinical development to broaden the armamentarium of therapeutic transcatheter mitral valve repair (TMVR) .
On the other hand, the feasibility of percutaneous mitral valve implantation (TMVI) in native valve anatomy has recently been reported in high-risk patients6-8, mainly with functional aetiology. The respective devices are currently under preliminary clinical evaluation.
With the development of TMVI technologies, the following questions arise. Will TMVI reduce the clinical value of the more complex repair approaches? Will percutaneous repair technologies progressively disappear in favour of replacement?
The question is relevant and goes beyond a theoretical discussion based on medical facts: the question as to WHO will perform the procedure may have an impact on HOW a specific patient is treated. Or, to be provocative, if an operator without specific training and profound understanding of the dynamic mitral valve and the underlying pathology is predominantly to perform these procedures, the pendulum will rather swing towards replacement. On the other hand, a tailored approach focusing on the underlying pathology will favour repair, particularly in patients where durability becomes important.
The debate will not be resolved quickly, since it is still ongoing after decades in the area of surgical mitral interventions.
What is the argument in favour of repair over replacement? It is mainly the safety profile (morbidity and mortality of the intervention) of repair techniques, which is higher compared to replacement. This element by itself should be strong enough, in our opinion, to favour repair whenever feasible. This becomes particularly true when treating lower-risk populations. Against repair is the unpredictability of the outcome, the complexity of some procedures, and the need for combining therapies based on the underlying pathology.
We believe that the question cannot and should not be “repair in all” versus “replacement in all”. The complexity of the mitral valve and the large variety of pathologies demand tailored approaches for each individual patient.
Beyond MitraClip: the portfolio of TMVR is rapidly increasing
The safety and efficacy of MitraClip therapy have been reported by several authors in both degenerative (DMR) and functional (FMR) aetiologies2,9,10. Periprocedural mortality is low, ranging from 1% to 6% even in very high-risk patients, and significant reduction of MR can be achieved in about 90% of cases2,10. The learning curve with this device is rather flat. Today, most patients can be treated with good outcomes in experienced hands, even in the presence of challenging anatomy (Barlow’s disease, presence of leaflet indentation, annular calcifications…)9,11.
Beyond MitraClip therapy, only a handful of devices have been introduced into clinical practice and are currently available for commercial use in Europe (CE marked). The concepts vary from percutaneous implantation of neochordae to annuloplasty devices and cinching techniques. All these devices proved to be safe in terms of periprocedural mortality4,5,12.
Table 1 summarises the features of the CE-marked devices for transcatheter repair of MR.
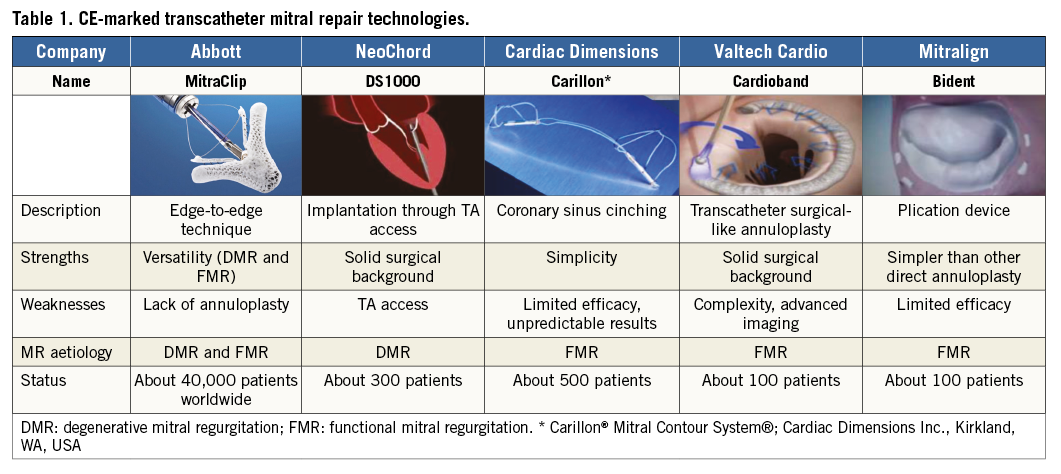
Combining different repair techniques will further expand the indication and improve efficacy and durability (i.e., leaflet repair in association with annuloplasty). Moreover, TMVR keeps the door open for other interventions in most cases. Different repair approaches can be combined in a single or staged procedure (i.e., combination of MitraClip and annuloplasty for both FMR and DMR; NeoChord [NeoChord, Inc., St. Louis Park, MN, USA] and annuloplasty for DMR). While it is today questionable to predict the possibility of TMVI following MitraClip, TMVI remains feasible after annuloplasty and probably also after NeoChord implantation.
Preliminary results of TMVI
Clinical experience with TMVI is still very limited. Currently, about 100 patients have been treated worldwide. Five different devices have been implanted in humans, and among them only four clinical programmes are active: the Tendyne (Abbott Vascular), the CardiAQ (Edwards Lifesciences, Irvine, CA, USA), the Twelve Intrepid (Medtronic, Minneapolis, MN, USA) and the Tiara™ (Neovasc Inc., Richmond, BC, Canada). The Edwards Fortis valve trial was prematurely terminated last year because of a high incidence of valve thrombosis.
Although a word of caution should be applied in the interpretation of data from early feasibility trials, with current TMVI devices the safety profile seems lower as compared to TMVR. Most devices showed very high 30-day mortality rates: 45% with the CardiAQ, 38% with the Fortis, 13% with the Twelve and 27% with the Tiara devices (Meredith I. Transcatheter Mitral Valve Implantation: Early Clinical Outcomes. EuroPCR 2016). In general, the high mortality observed with TMVI could be at least in part related to the fact that most of the patients treated were really sick and end-stage. The haemodynamic performance of successfully implanted devices was, however, convincing and accompanied by minimal rates of perivalvular leak. The outcomes observed with the abovementioned prostheses are detailed in Table 2.
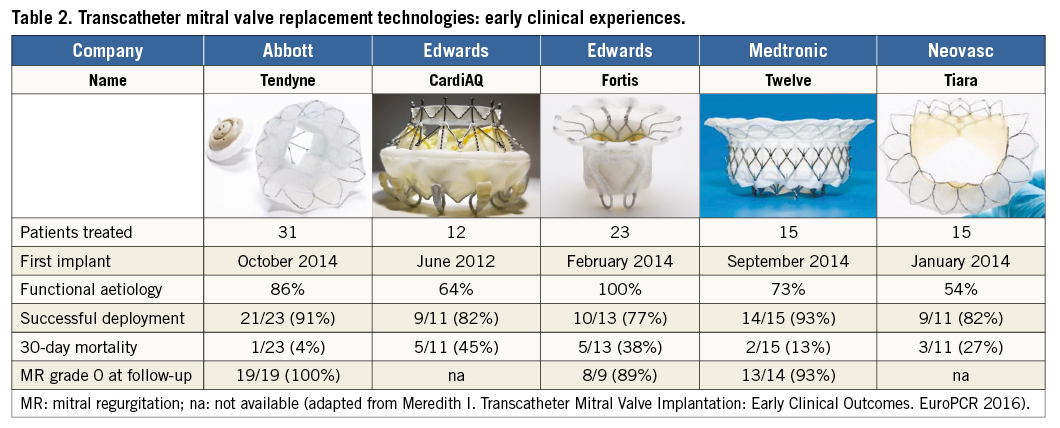
The device which for the moment has shown the best performance is the Tendyne prosthesis, which has the unique feature of full recapturability, and solid implantation with an apical tether. So far, 31 high-risk patients have been treated (86% with FMR). Thirty-day results have been reported for 23 patients: only one patient died due to septic shock (4%). All the patients had no MR at follow-up. Whether this excellent outcome is the result of careful patient selection or of a true design advantage is still a matter of discussion.
The technology of TMVI is currently hampered by the fact that only a minority of patients are eligible for these procedures (mainly due to anatomical limitations).
Specific advantages and disadvantages of transcatheter mitral valve repair and replacement
PHYSIOLOGICAL ASPECTS
The mitral valve complex is a sophisticated dynamic anatomo-functional structure, which is composed of multiple component features: the leaflets, the annulus, the chordae, the papillary muscles, and the continuity with the atrial wall and the aortic valve.
In general, a repair approach is more respectful of the physiology of the mitral valve complex. As shown in surgery, mitral replacement is associated with a non-physiological in-flow pattern from the left atrium to the left ventricle (LV). This results in increased LV stress and a loss in LV efficiency. This may not be trivial, in particular in patients suffering from heart failure with a low ejection fraction (EF). With replacement with large devices, reduced basal LV contraction is expected to be seen due to the fixation of the prosthesis to the mitral annulus. The contribution of basal contraction to the cardiac output again plays a major role in heart failure patients with severely depressed EF.
PROSTHESIS-RELATED FACTORS
The life expectancy of a patient implanted with a prosthetic valve is reduced, mainly due to thromboembolic and haemorrhagic events and to the risk of prosthetic valve-related endocarditis. Valve thrombosis represents a major issue in this initial phase of the development of TMVI. Some valves are implanted in a supra-annular position (more towards the atrium), inducing disturbed atrial flow dynamics, and risk of thrombosis. The Edwards Fortis clinical trial stopped patient enrolment to investigate this safety issue further, since evidence of valve thrombosis had been observed in some of the treated patients. All the patients undergoing TMVI most likely require long-term anticoagulation. Although at the moment no long-term data are available, it is likely that the duration of anticoagulation will be lifelong.
Durability and the possibility of structural valve deterioration should also be taken into consideration in the decision-making process. Surgical experience with tissue mitral prostheses has shown that durability is reduced. This is particularly true in younger patients.
Durability is a major issue also for mitral repair. Acute successful reduction of MR is fundamental to provide durable results in TMVR. The final results of the EVEREST II randomised trial showed that, when the acute procedural result is optimal, transcatheter mitral repair is durable13. However, in case of MR persistence or recurrence after mitral repair, outcomes are poor. This suggests that patients eligible for reparative procedures should be treated preferably in high-volume highly experienced centres.
PROCEDURAL ACCESS
The large majority of TMVI have so far been performed through transapical access in order to ensure coaxiality. Although the feasibility of transfemoral transseptal implantation has been reported with the CardiAQ prosthesis14, transapical access remains at the moment the most widely used approach. This represents a major disadvantage of TMVI compared to TMVR, since the transapical route is more invasive, is associated with more myocardial injury, requires general anaesthesia and in TAVI patients has been shown to be inferior to the transfemoral approach in terms of procedural outcomes15.
Different replacement devices delivered through a transseptal approach are currently under preclinical investigation. However, the need for transapical access remains at the moment a disadvantage of TMVI, since most of the TMVR technologies are delivered through transseptal access, or, as for the NeoChord, require a smaller apical access.
THE “CHIMERA” OF THE ONE-FITS-ALL CONCEPT
Specific advantages of replacement are versatility and more predictable results in terms of MR reduction. Moreover, while in order to address the different anatomies with repair techniques a single operator should be confident with multiple devices and advanced imaging guidance modalities, and technologies according to the anatomy and to the specific lesions, it can be expected that TMVI will be technically less demanding.
The concept that one replacement device could fit all the anatomo-functional mitral variations is appealing, but it is at the moment just theoretical. Patient eligibility represents a major issue for TMVI, and preoperative assessment with CT angio is mandatory to confirm anatomical feasibility. The most important factor, which limits patient eligibility, is the risk of LVOT obstruction due to the protrusion of the valve stent in the outflow tract. Today, up to 50% of patients are denied TMVI, mostly due to this issue.
Safety first!
Differently from early experience with TMVI, the MitraClip has shown excellent safety even in the early days of the initial learning curve. The reduced impact of repair compared to replacement in terms of physiology and anatomical alterations gives repair a higher safety profile, which is the fundamental background to expanding transcatheter mitral intervention indication towards an earlier indication. Early timing is crucial to achieve a substantial prognostic benefit, i.e., to restore life expectancy in DMR patients and obtain reverse remodelling in FMR patients. When patients are treated in too advanced a clinical status, any intervention becomes unable to influence the prognosis modifying the natural history of the disease.
Since the impact of mitral intervention is more effective when it is executed early, only a very safe procedure can justify a transcatheter therapy as a first-line option. If we consider safety and early indication, repair should be preferred to TMVI due to the lower acute risk and the lack of the long-term consequences of a prosthesis (including anticoagulant therapy, risk of structural valve deterioration and risk of infection).
Conclusions
Durability, safety and distortion of the physiology remain major concerns regarding TMVI as compared to TMVR. TMVI will therefore be a complementary therapeutic option for a great number of patients, especially in an advanced phase of the disease, with both DMR and FMR, who are not suitable for valve repair, while transcatheter mitral repair should remain in our opinion the first-line therapy whenever feasible.
In general, it could be stated that, in patients with DMR, TMVI will probably have a limited role only in high-risk, elderly and inoperable patients, who are not suitable for surgical mitral valve repair or with anatomical contraindication to transcatheter mitral repair. In patients with severe FMR, since even the role of surgery is less well established, and most patients are treated medically, TMVI may potentially be a therapeutic option for a large number of patients, especially with more advanced disease and severe anatomical and functional abnormalities, who are not eligible for valve repair.
Conflict of interest statement
F. Maisano is a consultant for Abbott Vascular and for Valtech Cardio. The other authors have no conflicts of interest to declare.

