Introduction
Coronary microcatheters (MCs) are often used in complex and chronic total occlusion (CTO) percutaneous coronary intervention (PCI)1,2 to facilitate guidewire manipulation and exchanges, and enhance their penetration force. Coronary MCs can be classified as high profile, low profile, angulated, dual lumen, and plaque-modifying1. Despite extensive clinical use, the failure modes of these devices have not been systematically studied. We queried the “Manufacturer and User Facility Device Experience” (MAUDE) database for reports on the most commonly used coronary MCs to understand their failure modes better.
Methods
The MAUDE is an online database created by the Food and Drug Administration (FDA) listing adverse events caused by approved medical devices. Reporting is either mandatory (for manufacturers and device user facilities) or voluntary (for healthcare professionals, patients, and consumers). We searched the database from January 2010 to January 2020 for reports on the most commonly used coronary MCs: Corsair and Corsair Pro (Asahi Intecc, Aichi, Japan), Caravel (Asahi Intecc), Finecross® (Terumo, Somerset, NJ, USA), and the Turnpike family: Turnpike®, Turnpike® LP, Turnpike® Gold, and Turnpike® Spiral (Teleflex, Wayne, PA, USA). The database was last accessed on 25 January 2020 by two independent reviewers (R. Sedhom and M. Megaly). The MAUDE database is publicly available and de-identified. Therefore, no institutional review board approval was required for this study.
The outcomes of the study included MC failure modes and their clinical consequences. Multiple mechanisms of failure were possible for each reported case. Categorical variables were described as numbers and percentages. All statistical calculations were performed with IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp., Armonk, NY, USA).
Results
A total of 467 reports were found during the study period. After the exclusion of peripheral interventions, duplicate reports, and unclear reports, our final cohort included 378 coronary MC events (Figure 1). Approximately 37% of the lesions were CTOs, with the retrograde approach used in 32.6% of those procedures (Supplementary Table 1).
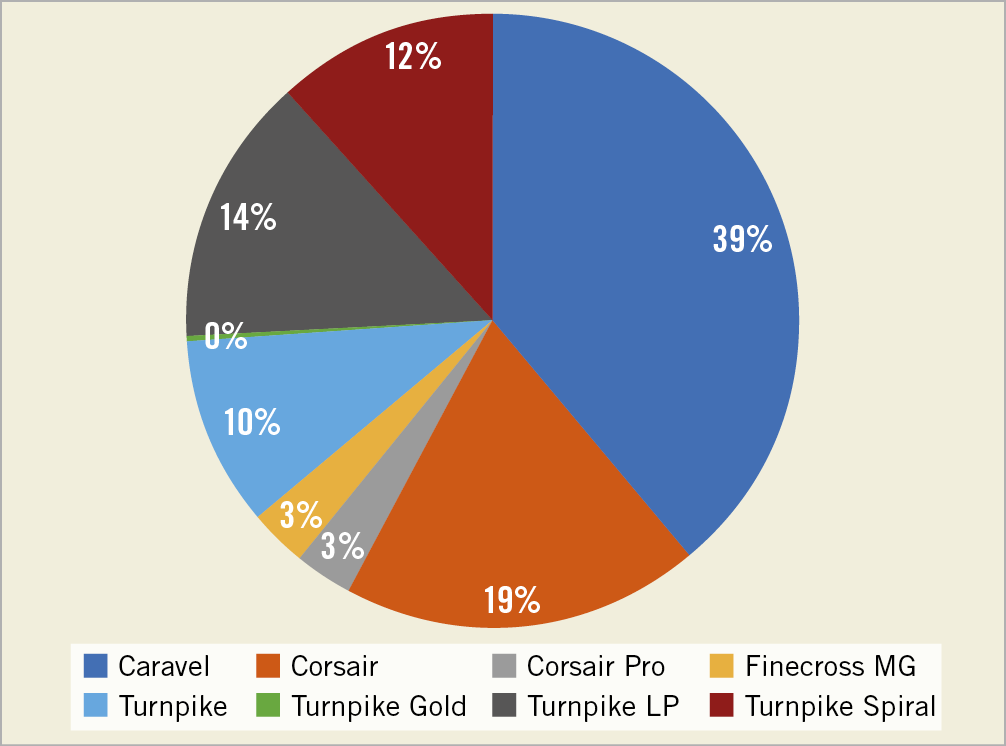
Figure 1. Reports of microcatheter failure in the MAUDE database.
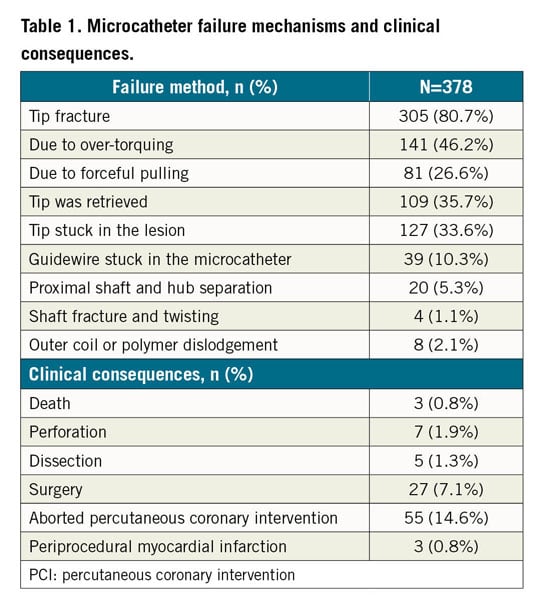
The most commonly reported failure mechanism was tip fracture (80.7%). Tip fracture was associated with over-torquing (46.2%) or forceful pulling of the MC (26.6%). The tip was retrieved in 35.7% of the cases. Other failure mechanisms included the MC tip getting stuck in the lesion (33.6%), the guidewire getting stuck in the MC (10.3%), proximal shaft and hub separation (5.3%), shaft fracture or twisting (1.1%), and outer coil or polymer dislodgement (2.1%) (Figure 2, Table 1). The most commonly reported clinical consequences of MC failure were aborted PCI (14.6%) and conversion to surgery (7.1%). The mechanisms of failure and clinical consequences of each microcatheter are shown in Supplementary Table 2.
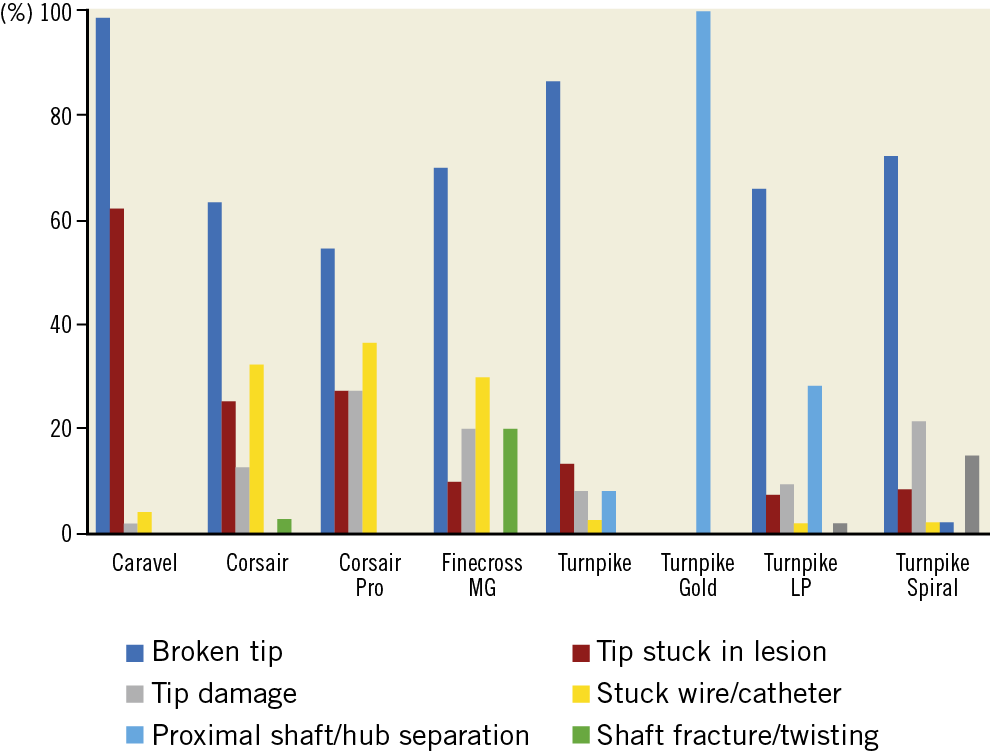
Figure 2. Failure modes of coronary microcatheters.
Discussion
Our study is the first to report systematically the failure modes of commonly used coronary MCs. The principal findings are that: 1) the most commonly reported MC failure mode was tip fracture (80.7%), most commonly due to over-torquing (46.2%) and forceful pulling of the MC (26.6%); 2) the primary mechanism of tip fracture of non-torqueable MCs (e.g., Caravel) was forceful pulling of the MC; and 3) the primary mechanism for tip fracture in high-profile MCs (e.g., Corsair) was over-torquing.
In our analysis, the most commonly reported failure mechanism was tip fracture secondary to over-torquing and forceful pulling of the MC after it became stuck in the lesion. It was most commonly observed in low-profile MCs, which have a weaker connection between tip and shaft. The tip was retrieved successfully in 35.7% of cases. Operators need to be familiar with the manufacturer’s instructions for use: low-profile MCs (e.g., Caravel) should not be torqued, as torquing may predispose to tip fracture. Such MCs should also not be used in heavily calcified lesions due to increased risk of tip entrapment3. When MC tip fracture occurs, attempts can be made for retrieval using various techniques4, such as snares or twirling guidewires. Alternatively, the fractured tip can be left in situ, which is frequently the preferred option5, with the lost tip often covered with a stent3.
Prolonged guidewire manipulation through an MC may result in guidewire entrapment. In our analysis, entrapment was reported in 33.6% of cases, mostly with large MCs. Flushing with saline before insertion may help to prevent this complication. If the guidewire starts feeling “sticky”, the MC should be replaced to avoid encasement within the MC, which may require removal of the entire system, resulting in loss of wire position.
Limitations
Our study is a retrospective analysis from the MAUDE database with the selection bias resulting from optional reporting by healthcare professionals. There is potential for significant underreporting of these events given the voluntary nature of disclosure. Second, the nature of the database limits the accuracy of the correlation between the device failure and clinical adverse events. Finally, the incidence of microcatheter failure cannot de determined, as the study lacks a denominator.
Conclusion
Coronary MCs are essential tools in contemporary PCI beyond CTO PCI. The most common failure mechanism reported in the MAUDE database was MC tip fracture due to over-torquing and forceful pulling. Operators should be aware of the limitations and mechanisms of failure of MCs in order to prevent malfunctions and be ready to manage them should they occur.
|
Impact on daily practice Our study is the first systematic report of coronary microcatheter malfunction. The most commonly reported MC failure mechanism was tip fracture, most commonly due to over-torquing and forceful pulling of the MC. We encourage the systematic collection of the frequency and type of microcatheter failure in prospective registries, which would allow determining the prevalence of microcatheter malfunction and optimal prevention and treatment strategies. |
Conflict of interest statement
S. Garcia reports being a consultant for Surmodics, Osprey Medical, Medtronic, Edwards Lifesciences, and Abbott, and receiving grant support from Edwards Lifesciences and the VA Office of Research and Development. M.N. Burke reports consulting and speaker honoraria from Opsens Medical, and being a shareholder in Egg Medical, and MHI Ventures. D. Karmpaliotis reports receiving speaker honoraria from Boston Scientific, Abbott Vascular, and Abiomed. E. Brilakis reports receiving consulting/speaker honoraria from Abbott Vascular, American Heart Association (associate editor, Circulation), Amgen, Biotronik, Boston Scientific, Cardiovascular Innovations Foundation (Board of Directors), CSI, Elsevier, GE Healthcare, Infraredx, Medtronic, Siemens, and Teleflex, research support from Regeneron and Siemens, and being a shareholder in MHI Ventures. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

