Abstract
Aims: This is the initial report of stent deformation / pseudofracture of the 7 crown Endeavor / Micro Driver stent platform (2.25-2.75 mm), whereby the post-dilation balloon catches and causes major stent deformation angiographically appearing as a large stent fracture. We sought to determine frequency and cause.
Methods and results: Analysis of 1,000 consecutive Endeavor/Micro Driver stents (7 crown) deployed at our institution. Bench testing was also performed by deploying 10 stents in 2.5 mm tubing and then attempting re-crossing with non-compliant balloons. Clinical results: There were 14 cases of major stent deformation/pseudofracture representing an incidence of 1.4% or 1.8% of 775 stents that were post-dilated. Of the 14 deformed stents, re-stenting was required in nine and MACE occurred in five (36%.). Benchtop results: Balloon “catch” was reproducible at an initial frequency of 9% and affected three of the 10 stents. With the use of provocative measures stent deformation occurred in 50% of test stents.
Conclusions: Stent deformation/pseudofracture of the 7 crown Endeavor/Micro Driver platform occurred in 1.8% of cases that were post-dilated. Of these, 36% experience MACE. Deformation can be reproduced on the benchtop. Operators should be aware of the complication, and develop strategies to deal with it.
Introduction
The Endeavor zotarolimus eluting stent (Medtronic, Santa Rosa, CA, USA) is based on the Driver/Micro Driver platform and became available in Europe in 2005 after the Endeavor I study1, and was approved by the US Food and Drug Administration in October 2007. Medtronic’s newer generation Resolute drug-eluting stent (DES) utilises the same drug and stent platform, but incorporates a new polymer.2
The Driver/Micro Driver platform is a cobalt-based alloy stent with thin rounded struts. The Endeavor Sprint and Resolute 2.25mm to 2.75 mm stents are based on the Micro Driver, which has 7 crowns and, importantly, a single helix backbone (Figures 1 and 2). Stents 3 mm in diameter and larger are based on the Driver stent, which has 10 crowns and a double helix backbone. This offers more longitudinal stability, but potentially less flexibility. The open cell design of the Driver platform also results in the largest stent cell area of commonly used DES which should allow easy side branch access.3
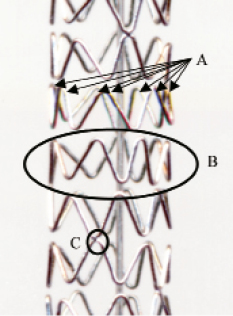
Figure 1. Basic structure of Micro Driver/Endeavor 7 crown stent. The stent is made of modules or rings (A) which are each approximately 1.2mm in length. Each module has 7 crowns (B). The modules are joined by a single weld (C). Weld pattern is shown in Figure2.

Figure 2. Schematic representation of the weld points in a 7 Crown stent. Modules (stent rings) are welded by a single crown to their adjacent module (except the first module at each end which has two attachment points). The position of the weld point rotates to create asingle helix “backbone”.
The Endeavor stent was introduced into the Australian market in October 2006, and became one of two preferred DES at our institute in July 2007. Prior to the introduction of the Endeavor stent, the use of the Micro Driver was very limited at our site due to market competition surrounding bare metal stent (BMS) use.
The use of the Endeavor stent has been significant (over 1,500 Endeavor DES deployed to date), however, balloon “catching” and major stent deformation, angiographically appearing as a large stent fracture, has been observed following post-dilation of the 7 crown stent at our institute. In this report, we detail our series of stent deformations, with particular focus on frequency and cause, and postulate mechanisms and techniques for preventing this occurrence.
Methods
Procedural data on all PCIs conducted at our institute is collected real time with prospective follow-up using an ethics approved outcome study protocol. Procedural, lesion and stent details are stored in a relational database. Follow-up data at 30 days, six months and one year including MACE and drug compliance is also collected. There is mandatory local reporting of potential device failure. After two cases of stent deformation were reported to the Medical Advisory Committee of the clinic, information was sent to all operators warning of these events.
Initial data suggested this to be a rare event, but after further reported events it was elected to analyse all 7 crown Endeavor/ Micro Drivers deployed from the time of first notification. Sample size was set at 1,000 consecutive stents with the expectation that this could be achieved within a reasonable time frame. A pseudo-fracture was deemed present when the following occurred. Firstly, the operator felt significant catch when trying to pass or was unable to pass a post-dilation balloon. Secondly, acquisition without contrast needed to show some “gapping” of the stent, confirmed by an angiogram with contrast showing lack of vessel support at that site. Finally, these findings needed to be confirmed by at least two other authors. Reporting of events was prospective.
PCI procedures were typically performed from the femoral approach using 6 or 7Fr sheaths. Standard equipment included EBU or Judkins right guides, Balance Middle Weight or High Torque Floppy (Abbot Vascular, Santa Clara, CA, USA) wires and Sprinter Legend (Medtronic, Santa Rosa, CA, USA) pre-inflation balloons. The stent was chosen to match the size of the artery or the vessel at the distal edge if it tapered. Deployment pressure and stent size is shown in Table1. When stents were post dilated, commonest balloons used were NC Sprinter (Medtronic, Santa Rosa, CA, USA), NC Mercury (Abbot Vascular, Santa Clara, CA, USA) and Sapphire (OrbusNeich, Hoevelaken, The Netherlands). Balloon length would always be shorter than stent length and nominal size typically 0.5mm larger (equating with a 0.25 mm upsize at 21 atm).
Initial events were reported to Medtronic and to the Therapeutic Goods Administration (local equivalent of FDA). Enrolment began July 2007 and 1,000 stents were enrolled by January 2010. Demographic, angiographic and procedural data was analysed. Bifurcations treated with two stents or kissing inflations were excluded. Statistical data was analysed using a commercial biostatistics package (Instat; GraphPad Software Inc., La Jolla, CA, USA) using Chi-square for categorical values and unpaired t tests for comparative data.
Simultaneously, bench testing was conducted independently of similar Medtronic tests. Ten 7 crown stents, 18mm length (five Micro Drivers, five Endeavor Sprints) were deployed using semi-compliant clear vinyl tubing with a diameter of 2.5mm at a constant radius curve of 50mm diameter. Stents ranged from 2.25 to 2.75mm in diameter and were deployed at 12, 16 or 20 atmospheres in order to mimic under/oversizing and gentle/aggressive deployment pressure. The stents were then imaged using IVUS and optical external scanning using a modified flatbed scanner. Attempts were then made to reproduce the clinical scenario by crossing with a variety of contemporary and historical non-compliant balloons, specifically 13 or 15mm length NC Sprinter, NC Mercury, PowerSail (Guidant, Santa Clara, CA, USA) and Sapphire. Balloon size was stent diameter +0.25 or 0.5mm. Each stent was crossed 10 times (three times each with NC Sprinter and NC Mercury, then twice with PowerSail and Sapphire) and the incidence of catch noted. Provocative manoeuvres were then performed (use of different wires, changing aspect of stent, increasing bend and increasing profile of balloon/tip).
Results (clinical)
Fourteen (14) stents developed the typical features of difficulty with re-crossing then angiographic stent separation and pseudofracture. This represents an incidence of 1.4% of all 7 crown stents deployed. Given that stent separation can only occur with post-dilation, a more meaningful incidence may be 1.8% (14 of 775 stents where post- dilation was attempted)
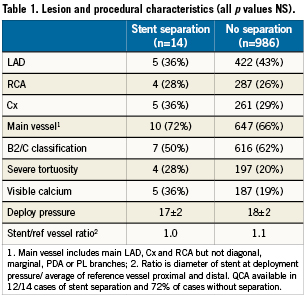
Lesion and procedural characteristics are shown in Table1. Vessels that suffered stent separation trended towards more calcium, tortuosity and lower stent to reference vessel ratio. The differences in deployment pressure, lesion site and classification were minor.
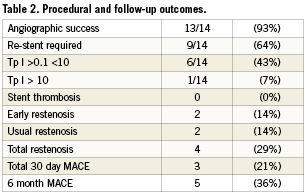
Outcome (procedural, short and mid-term) data is shown in Table2. In order to achieve a high angiographic success rate in 13/14 cases (93%) the gap section of the fractured stent was bridged with another stent in 9/14 (64%) of cases. In the five cases where the pseudofracture was not re-stented, 30 day MACE occurred in three cases. In the first of these cases, a marginal Cx stent could never be reliably re-crossed. There was a distal dissection that appeared stable. It was thought unlikely that a further stent could be safely delivered hence the vessel was not re-stented. Distal vessel occlusion resulting in NSTEMI occurred within 24 hrs. In the other two cases that were not re-stented, early “restenosis” occurred at the site of stent fracture where the vessel was left unsupported. Both cases presented with angina without troponin (Tp) elevation within 30days.
Bench testing
During initial bench testing, unprovoked balloon catch was felt during nine of 100 passes affecting three of 10 stents. With provocative manoeuvres a total of five stents (50%) could be deformed. Detailed results are shown in Table3. Provocative factors included stiffer wires which increased wire bias, slower speed of balloon advancement, orientation of stent in tubing such that the tip of the post-dilatation balloon came in contact with the stent opposite its supporting helix, increased angulation of the bend and post dilating a short segment to create a non-cylindrical shape. Older model balloons were not associated with an increased incidence. Figure2 shows the typical stages of stent deformation. Of note is that the strut that became caught on the balloon appeared to be pushed under the struts distal to it, causing separation proximal to the point of impact and an area of trauma just distal to the separation; this result was also seen clinically. External optical scanning/photography as shown gave a better overview of deformation than IVUS.
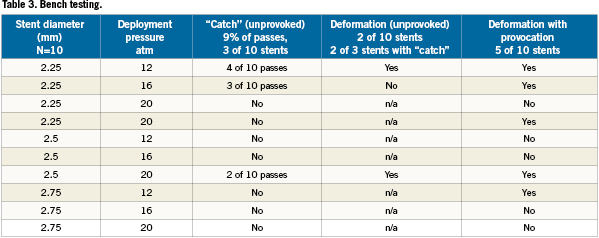

Figure 3. Typical stages of stent deformation when a post-dilatation balloon catches. Panel A shows mild stent deformity with the “caught” strut pushing under distal strut. In panel B, there is now “crowding” of the distal stent and minor separation proximally. The stent begins to separate in Panel C, and major stent deformity is seen in Panel D. Note deformation of the module which may explain difficulty in re-crossing.
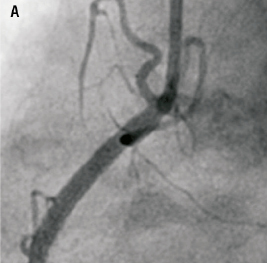
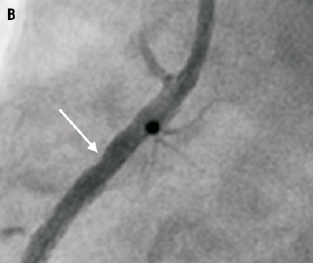
Figure 4. Usual angiographic appearance of the stent post delivery (Panel A) and after balloon catch and deformation (Panel B). Note the appearance of a new residual stenosis (arrow) at the site of pseudofracture where the artery is now unsupported.
Discussion
Deformation of the 7 crown Endeavor stent has been observed at our institute and is reproducible in a bench top model. Given that this stent platform has been in use for many years, it is unclear as to why this complication has not been previously noted. Three possibilities were considered.
Firstly, this complication may have existed for some time but remained unrecognised. Examples would include late stent thrombosis with DES that was not acknowledged until the letter of Ong4, thereafter every large study of DES has observed this complication. Similarly, Cypher stent fracture was initially not well described but is now recognised to occur relatively frequently5,6.
Secondly, this phenomenon may have been partially recognised by other institutes, but not named or properly described e.g., the Takotsubo phenomenon. This condition has probably always exist yet was only first documented in 1990 (in Japanese) and then 1991 by Sata el al7. It is now commonly recognised.
Finally, the problem may be related to particular practices at our institute (e.g., relatively high stent deployment pressure followed by post-dilation).
Which of these explains the findings of this study remains unclear. Our institute does deploy stents at relatively high pressures as a result of previous routine use of IVUS for all stent restenosis and early recognition of the importance of correct deployment. Since then median stent deployment pressure has increased from 14atm to 18 atm and post-dilation pressures have also risen to a median of 21 atm, with 30% of stents post dilated at pressures >25atm. Additionally, the frequency of post-dilation is high (70%). Although unproven, it is our belief that the practice of both high pressure deployment and then additional post-dilatation with a shorter, larger balloon may be unusual and may explain the occurrence of this complication at this institute. Longitudinal balloon growth by the deployment balloon may be implicated, but to date we have been unable to prove this.
Intuitively, stent length and sizing may affect the frequency of this phenomenon. Under sizing would result in the stent not being opposed to the wall and thus more likely to allow balloon catch. Longer stent length would also present the post dilation balloon more opportunity to catch. All clinical cases of stent deformation were reviewed and stent under sizing was implicated in one case only where a long 2.25mm stent was deployed to match the distal vessel and was undersized proximally with subsequent stent deformation. Bench testing did not specifically address this issue, but it is notable that the smallest stent deployed at the lowest pressure was the most easily deformed.
The clinical significance is also unclear as the frequency of stent deformation was relatively low (1.8%) but MACE in these cases relatively high (35%). The low incidence was partially due to the strict criteria for deeming deformation to have occurred (as outlined in the methods section). Additionally, operators learnt to not persist with non-compliant balloons if “catch” was felt, rather a non-compliant balloon with a more favourable nose cone was likely to be used to restore stent apposition and several cases of stent deformation were likely avoided.
Although we did not see stent thrombosis, operator persistence and the high frequency of “repair” with an additional stent lead to good angiographic results in the majority of cases. It is plausible that if left with unrepaired stent deformation, probable poor apposition and an unsupported segment of artery within the stent, then thrombosis would be a complication, especially if the unsupported section had dissection. This would lead to an increase in mechanical early stent thrombosis and it is noteworthy that Endeavor III8, Endeavor IV9, Sort Out III10, Zest11 and most recently the RESOLUTE All Comers trial12 all lend some support to that hypothesis with a signal of early stent thrombosis seen in the Endeavor arm.
Although angiographically this can look like a stent fracture, in the bench model, stent deformation was seen to be due to “pseudofracture” i.e., the modules do remain connected but become deformed. The micro-CT images of Basulus et al3 refer to the 10 crown Endeavor stent rather than the 7 crown but do show large stent cell areas and very large ring to ring measures (3.33 mm vs. 1.87 for XienceV). Looking at these micro-CT images, especially Figure 6A of that study, in addition to Figures1 and 2 of our study, it is plausible that the Endeavor would have limited longitudinal stability.
The phenomena was relatively easy to replicate under “worst case” conditions as detailed in results. The module that the balloon became trapped on was seen to be pushed distally, but then distort and rotate longitudinally thus trapping the wire and making further crossing difficult. This rotation should be prevented by additional links between the modules and probably explains why stent separation was not seen in the 10 crown stents which have a double helix connecting backbone rather than a single helix.
Benchtop work has shown that once the deformation begins, a vicious cycle begins whereby the balloons become harder to pass and become more likely to cause further deformation. In view of this, we recommend that if operators feel resistance to the passage of post dilatation balloons within such a stent, forceful manoeuvres are strongly discourage. It has been our practice to assume that the stent may be undersized and go back with a suitably sized low profile complaint balloon at moderate pressure (16 atm) and then proceed with a non-compliant balloon if required. The incidence of mild catch requiring operator to change to a different post dilation strategy is estimated at 10% (as assessed by a database audit of the frequency of a compliant balloon being opened after stent deployment) suggesting that the true incidence of minor stent deformation may be significantly greater than 1.8%. Minor forms of stent disruption would be difficult to see angiographically and better felt as an inability to pass balloons freely through the stent.
If a stent is deformed, it is not unusual to find it difficult to re-cross with any balloon. We have a low threshold to change down to small diameter balloons and increase diameter in step wise fashion until balloons pass easily. Based on our bench work, we believe the artery is unsupported at the site of separation and would recommend an additional stent to bridge the gap.
Limitations
The results of this study pertain to a single centre only and should be interpreted cautiously unless further sites subsequently report similar complications. Additionally, the phenomenon may be related to specific stent deployment technique (high inflation pressures, high incidence of post dilation) and may not be relevant to all sites. Finally, it is impossible to replicate every clinical scenario on the bench so it is unknown if certain post dilation balloons or balloon lengths cause more problems than others.
Conclusions
We have observed major stent deformation of the Medtronic 2.25-2.75mm (7 crown) stent with the attempted passage of post deployment balloons in 1.8% of cases. MACE occurred in 35% of these. It is unclear as to why this complication appears unique to our institution. Benchtop and clinical experience show that if balloons are difficult to pass, initial forceful manoeuvres are discouraged as this tends to make deformation worse. Operators need to be aware of the complication and develop strategies to overcome it. We did not see early stent thrombosis but benchtop modelling has shown that deformation would provide the substrate for early stent thrombosis.
Conflict of interest statement
The authors have no conflict of interests to declare.

