Abstract
Aims: Recent reports have suggested susceptibility of novel thin-strut coronary stents to incur longitudinal stent deformation during or following deployment. This analysis assesses the incidence of longitudinal stent deformation in three stent platforms.
Methods and results: Quantitative angiographic analysis (QCA) of 2,403 stents from the PERSEUS Workhorse (WH) and PLATINUM-WH trials was performed by an independent core laboratory. The distribution of QCA measured: nominal stent length ratios was compared between platforms to evaluate longitudinal stent deformation. Stent length ratio averaged 0.95±0.07 in the TAXUS Express arm and 0.94±0.06 in the ION (TAXUS Element) arm of the PERSEUS-WH trial (p=0.16). In the PLATINUM-WH trial, the mean ratio in the PROMUS cohort was slightly smaller (0.92±0.09) than PROMUS Element (0.94±0.08; p=0.004). Manual, blinded re-examination by the core laboratory of the angiograms corresponding to the 20 lowest and highest ratios in each trial did not identify any cases of stent deformation.
Conclusions: Systematic analysis by an independent angiographic core laboratory of 2,403 stents implanted in patients enrolled in two large multicentre randomised trials demonstrated no stent deformation or meaningful differences in stent length ratios between ION and TAXUS Express or between PROMUS Element and PROMUS in the patient populations studied using the methodology employed.
Abbreviations
CoCr: cobalt chromium
DES: drug-eluting stent
PtCr: platinum chromium
QCA: quantitative coronary angiography
WH: workhorse
Introduction
Coronary stent platform characteristics may influence both periprocedural and late performance measures1-3. Observations from preclinical studies and clinical trials suggest that stent design also impacts the vascular response to stent deployment4-8. Stent strut orientation as well as cell design (open versus closed) and stent type (multicellular, slotted tube, coil, self-expanding) influence the degree of stent-induced platelet activation as well as the magnitude of inflammatory neointimal response4,7,9. Similarly, both stent strut thickness and metal alloy composition have been shown to affect the degree of endoluminal stent-mediated injury and/or the consequent degree of inflammatory response10,11. Both randomised trials and registry reports have demonstrated a direct relationship between stent strut thickness and late restenosis, which may be most evident in smaller vessels12-18.
In an effort to enhance stent procedural performance parameters of profile, flexibility, radial strength and visibility, novel thin strut cobalt chromium (CoCr) and platinum chromium (PtCr) coronary stent platforms have been developed. The PtCr alloy has demonstrated increased bend fatigue resistance, greater conformability and similar radial strength versus 316L stainless steel stents and less stent recoil when compared with a CoCr stent platform2. Because PtCr is more dense than 316L stainless steel, radiopacity is enhanced3. Preclinical testing has supported the vascular compatibility of PtCr, with more rapid stent strut coverage and endothelialisation compared with 316L stainless steel6,8.
Based on the manner in which two adjacent rings of a stent are oriented and connected to each other, stent designs can be classified into four basic categories (peak-to-valley/in-phase; peak-to-peak/out of phase; mid-strut connector; offset peak-to-peak) which may influence longitudinal stent strength and deformation/compression resistance19.
The PtCr ION (TAXUS Element, Boston Scientific, Natick, MA, USA) paclitaxel-eluting stent (offset peak-to-peak) has demonstrated comparable safety and efficacy to the 316L stainless steel TAXUS Express stent (Boston Scientific) (peak-to-valley/in-phase) at 1- and 2-year follow-up in the Prospective Evaluation in a Randomized Trial of the Safety and Efficacy of the Use of the TAXUS Element Paclitaxel-Eluting Coronary Stent System for the Treatment of De Novo Coronary Artery Lesions (PERSEUS) trial20. Furthermore, the PtCr Promus Element (Boston Scientific) (offset peak-to-peak) everolimus-eluting stent (EES) was demonstrated to be non-inferior to the predicate CoCr PROMUS/XIENCE (Boston Scientific) (peak-to-valley/in-phase) EES for target lesion failure to 1-year follow-up and safety measures were similar in the Prospective, Randomized, Multicenter Trial to Assess an Everolimus-Eluting Coronary Stent System (PROMUS Element) for the Treatment of up to Two De Novo Coronary Artery Lesions (PLATINUM) trial21. Recent reports have suggested a susceptibility of several thin-strut coronary stents to incur longitudinal deformation during or following deployment22,23. Although reported cases describe a “concertina-like” stent strut overlap (compression), “pseudofracture” or gaps between struts due to longitudinal stretch have also been reported. Reports to date have come from bench testing or clinical anecdote without systematically examining the issue using objective criteria19,22-27. As such, no data exist from which to estimate the frequency or correlates of occurrence for longitudinal stent deformation. In this context, the quantitative coronary angiography (QCA) database from the PERSEUS and PLATINUM trials provides a unique opportunity to analyse both the incidence as well as factors associated with longitudinal stent deformation across three distinct coronary stent platforms with differing metal alloy composition (316L stainless steel, CoCr, PtCr), strut thickness (132 µm, 81 µm) and stent design.
Methods
This post hoc analysis examined patients in the PERSEUS Work Horse (WH) and PLATINUM-WH trials who received only a single study stent (patients receiving multiple stents were excluded to eliminate confounding from stent overlap). QCA of post-procedure angiograms was mandated by the study protocols in all patients and the same angiographic core laboratory was utilised for both trials. For each stent, stent length measured by QCA was compared to the nominal stent length. A ratio of 1.0 would indicate equivalent measured and nominal stent lengths. For each trial, the distribution of measured versus nominal stent length was compared in the test and control arms.
Subject selection, procedure, and follow-up
The PERSEUS-WH trial design has been described previously20,28. Briefly, the PERSEUS-WH trial was a prospective, randomised (3:1), single-blind controlled trial designed to evaluate non-inferiority of the PtCr ION (TAXUS Element) stent compared with the TAXUS Express2 paclitaxel-eluting stent. Subjects were eligible for enrolment if they required treatment of a single, de novo target lesion of ≥50% diameter stenosis with length ≤28 mm and reference vessel diameter ≥2.75 to ≤4.0 mm in a native vessel. Two-year follow-up has been completed29 and further follow-up will be conducted yearly for a total of five years.
The PLATINUM-WH trial has also been reported previously21. Patients who were ≥18 years of age with stable or unstable angina pectoris or documented silent ischaemia and required intervention of one or two de novo native coronary artery target lesions with RVD 2.5 to 4.25 mm, lesion length ≤24 mm, and diameter stenosis ≥50% to <100% with Thrombolysis In Myocardial Infarction (TIMI) flow grade 2 or 3 (by visual estimate) were considered eligible for enrolment. Enrolled patients were randomised (1:1) to receive either the PtCr PROMUS Element everolimus-eluting stent or the CoCr everolimus-eluting PROMUS stent. One-year follow-up has been reported21, and further follow-up will be conducted yearly for a total of five years.
Device description
The TAXUS Express2 stent platform (Boston Scientific) consists of the balloon-expandable Express Stent with controlled, slow release of paclitaxel at a dose of 1 µg/mm2 from a poly(styrene-b-isobutylene-b styrene) polymer. The Express Stent is laser-cut from 316L stainless steel tubing into an architecture of alternating, connected large and small rings with a peak-to-valley/in-phase design and three links connecting adjacent rings19. Stent strut thickness is 132 µm.
The PtCr ION (TAXUS Element) stent (Boston Scientific) is laser cut from a tube of platinum chromium alloy28. The characteristics of the drug-polymer system of the PtCr ION stent have similar kinetics to those of the previous generation TAXUS Express30,31 and Liberté16 316L stainless steel stent platforms28. The Element platform has an offset peak-to-peak design with two links connecting adjacent rings19. Stent strut thickness is 81 µm.
The Multi-linkVision stent underlies the CoCr PROMUS/Xience V platform and consists of serpentine rings in a peak-to-valley/in-phase design connected by three links fabricated from a single piece of medical grade L-605 cobalt chromium alloy19,32. Stent strut thickness is 81 µm.
The PtCr PROMUS Element everolimus-eluting stent (Boston Scientific) is comprised of the same Element stent platform as utilised in the PtCr ION paclitaxel-eluting stent. In PROMUS Element, the antiproliferative agent everolimus33 (100 µg/cm2) is applied in a biocompatible acrylic polymer and fluorinated copolymer identical to that used in the CoCr PROMUS (XIENCE V)32, manufactured and distributed by Abbott Vascular, Santa Clara, CA, USA as Xience V and distributed as PROMUS by Boston Scientific Corporation, Natick, MA, USA.
Quantitative coronary angiography
Standard image acquisition was performed at the clinical sites using two or more angiographic projections of the stenosis, intracoronary nitroglycerine to provide maximum coronary vasodilation, and repetition of identical angiographic projections of the lesion at baseline and final angiography. Angiograms were forwarded to Beth Israel Deaconess Angiographic Core Laboratory (Boston, MA, USA) for independent review. Using the contrast filled injection catheter as the calibration source, quantitative angiographic analysis was performed using a validated automated edge detection algorithm (Medis CMS, Leiden, The Netherlands)34. Selected images for analysis were identified using angiographic projections that demonstrated the stenosis and stent in an unforeshortened view, and minimised the degree of vessel overlap. The angiographic projection that minimised stent foreshortening was used for analysis. The core laboratory performed an additional manual review of the patients with the 20 highest and lowest QCA measured:nominal stent length ratios in each trial in order to assess whether the high or low ratio was due to longitudinal compression/elongation or some other cause. For this visual assessment, longitudinal compression/elongation was defined as inconsistency in the radiodensity pattern along the length of the stent, or other gross irregularities or deformities.
Statistical methods
Descriptive statistics are reported as mean±SD or n/N (%). Statistical analyses were performed using SAS statistical software, version 9.2 (SAS Institute, Cary, NC, USA). The ratio of QCA measured to nominal stent lengths were compared within each trial with a student’s t test. The ratio of measured to nominal stent length was evaluated in the overall population of patients who received a single study stent and in the subset of patients who received post-dilatation. P-values <0.05 were considered statistically significant and were 2-sided.
Results
This analysis included the 1,152 patients from the PERSEUS-WH trial (TAXUS Express n=289; PtCr ION n=863) and the 1,251 patients from the PLATINUM-WH trial (CoCr PROMUS n=612; PtCr PROMUS Element n=639) who received a single study stent. No instances of stent deformation were reported by the investigators in the PERSEUS-WH or PLATINUM-WH trials.
Patient and lesion characteristics
Baseline patient and lesion characteristics were generally well-matched between treatment arms within the PERSEUS-WH and PLATINUM-WH trials examined in this analysis (Table 1). Patients receiving TAXUS Express stents were older (63.6±9.5) and were more likely to have hypertension requiring medication (81.6%) than those receiving PtCr ION stents (62.2±9.6 years old, p=0.02; 74.8% with hypertension, p=0.02). Among PLATINUM-WH patients, the proportion of patients with medically treated diabetes was higher in CoCr PROMUS (PROMUS 25.0% vs. PROMUS Element 20.3%, p=0.049) whereas lesion length was longer in PtCr PROMUS Element (12.6 mm versus 11.9 mm in PROMUS; p=0.008) treated patients.
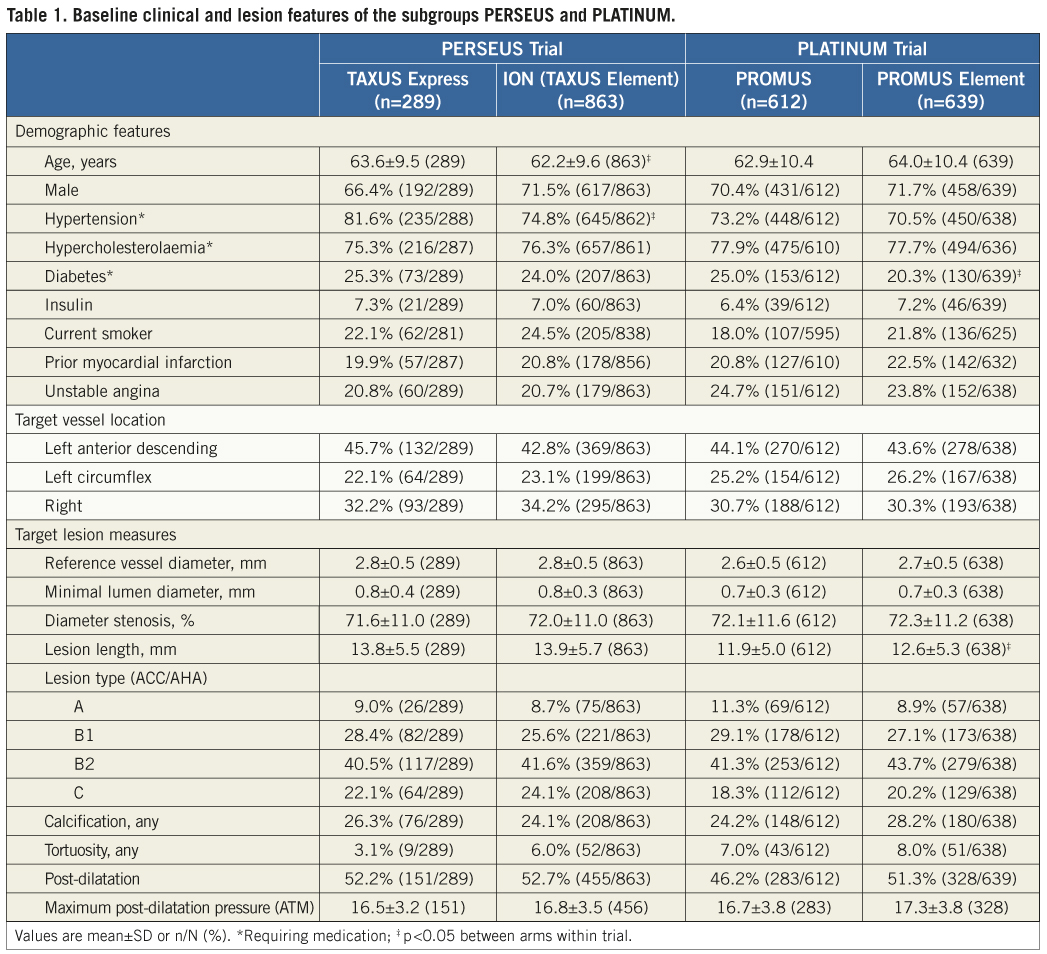
Measurement of QCA stent length: nominal stent length ratios
The distribution of the ratio of measured to nominal stent length was examined in both arms of the PERSEUS and PLATINUM trials. Mean stent length ratios were 0.95±0.07 in the TAXUS Express arm and 0.94±0.06 in the PtCr ION arm of the PERSEUS-WH trial (p=0.16; Figure 1A). The maximum and minimum values in each treatment cohort were similar (maximum: TAXUS Express 1.28 vs. ION 1.23; minimum: TAXUS Express 0.61 vs. ION 0.59; Figure 1A). Analysis of those patients who received post-dilatation (TAXUS Express n=151; PtCr Ion n=455), demonstrated very similar outcomes. Mean stent length ratios were 0.95±0.08 in the TAXUS Express arm and 0.94±0.07 in the PtCr ION arm of the PERSEUS-WH trial (p=0.10; Figure 2A).
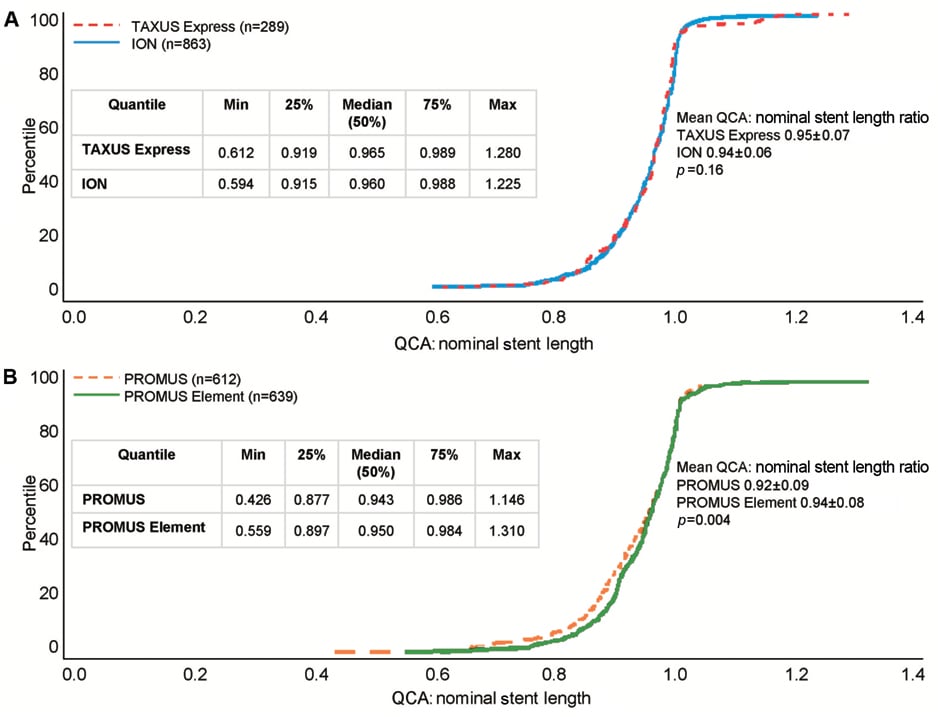
Figure 1. Distribution of ratios of QCA stent length to nominal stent length in the PERSEUS and PLATINUM trials. Cumulative distribution function of the ratio of measured stent length by QCA to nominal stent length in the (A) PERSEUS-WH (TAXUS Express dashed red line; ION solid blue line) and (B) PLATINUM-WH trials (PROMUS dashed orange line; PROMUS Element solid green line).
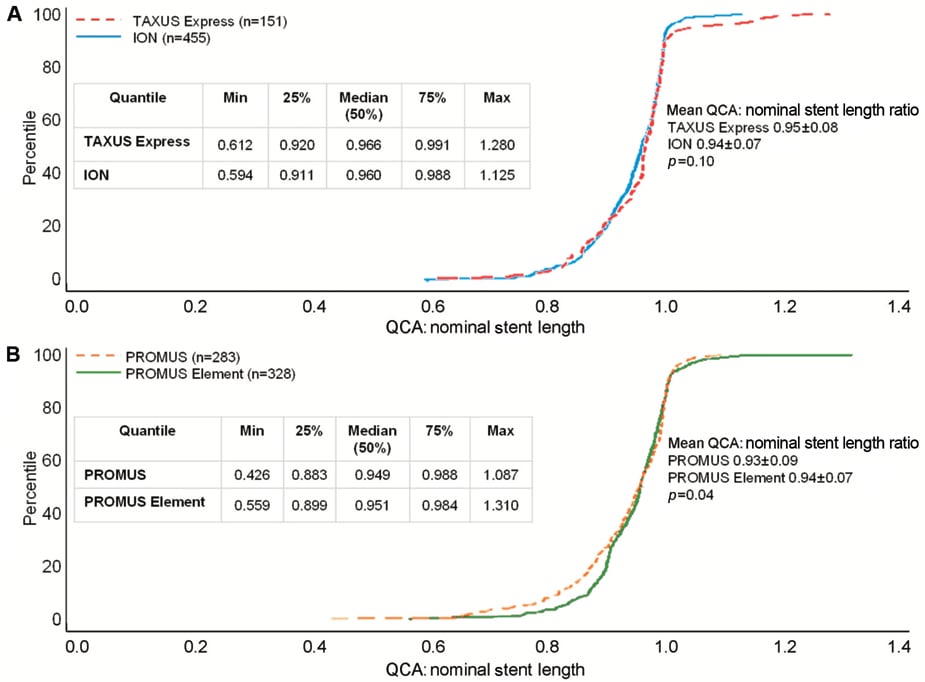
Figure 2. Distribution of ratios of QCA stent length to nominal stent length in patients receiving post-dilatation in the PERSEUS and PLATINUM trials. Cumulative distribution function of the ratio of measured stent length by QCA to nominal stent length in patients receiving post-dilatation in the (A) PERSEUS-WH (TAXUS Express dashed red line; ION solid blue line) and (B) PLATINUM-WH trials (PROMUS dashed orange line; PROMUS Element solid green line).
In the PLATINUM trial, the cumulative distribution curves of stent length ratio were similar in the CoCr PROMUS and PtCr PROMUS Element arms. The mean of the distribution of ratios in the PROMUS cohort was slightly smaller (0.92±0.09) than PROMUS Element (0.94±0.08; p=0.004, Figure 1B).The maximum stent ratio was 1.15 in PROMUS and 1.31 in PROMUS Element and the minimum stent ratio was 0.43 in PROMUS and 0.56 in PROMUS Element (Figure 1B). In patients in whom post-dilatation was performed (PROMUS n=283; PROMUS Element n=328), the mean ratio in the PROMUS cohort was 0.93±0.09 compared with 0.94±0.07 in PROMUS Element patients (p=0.04; Figure 2B).
Manual blinded re-examination of the angiograms corresponding to the 20 lowest and highest ratios measured by the core laboratory for each stent type did not identify any cases of stent deformation. Very low or high measured:nominal ratios appeared to result from inherent QCA variability and out-of-plane magnification from the calibration source. Low ratios were also at times observed in patients where the longitudinal axis of the stent was not orthogonal to the imaging beam (i.e., foreshortened view).
Discussion
The objective of this analysis was to assess the incidence of longitudinal stent deformation in multiple stent platforms. We found: (1) stent deformation was not observed in this study of selected patients with any of the three stent types and, (2) no substantial differences in QCA measured:nominal length were found between comparator stents in the overall patient populations or in the subset of patients receiving post-dilatation.
Following the advent of drug-eluting stents (DES), the practice of coronary stenting has evolved towards the use of longer stents and/or the treatment of more complex lesion morphologies35,36. This trend has been facilitated by the development of thinner strut, more flexible and deliverable stent platforms. In addition to a reduction in stent strut thickness, specific design iterations have included a reduction in the number of fixed connectors and/or their longitudinal distribution between adjacent expandable ring elements to enhance device flexibility and conformability2,19.
PROMUS Element stent conformability was reflected in the analysis of the intravascular ultrasound cohort of the PLATINUM QCA trial which demonstrated a low rate of incomplete stent apposition post procedure compared to historical controls37. Changes in stent metal alloy composition have also allowed thinner strut devices to maintain or enhance radial strength and visibility compared with 316L stainless steel2-4. Indeed, enhanced stent platform visibility and/or improvements in the stent delivery system were associated with a reduction in the requirement for unplanned (“bail out”) stent deployment and a lower incidence of “geographic miss” following PtCr PROMUS Element versus CoCr PROMUS stent deployment in the randomised PLATINUM trial21. In addition, enhanced radial strength and/or reduced stent recoil may explain the larger post-deployment in-stent minimum lumen diameter despite a similar deployment pressure and frequency of post-deployment dilatation following PtCr ION versus the stainless steel TAXUS Express stent in the PERSEUS randomised trial20.
However, recent reports question whether these structural stent platform iterations may have had a deleterious impact on longitudinal stent strength and stability. Specifically, cases of angiographically evident longitudinal stent deformation have been reported following deployment in patients of at least eight different stent types which encompass a variety of alloys, stent strut thicknesses and designs (Table 2)22-24,27,38. Typically, these anecdotal reports have been related to procedural circumstances, including stent implantation in diffusely diseased, tortuous and/or fibrocalcific lesions39.
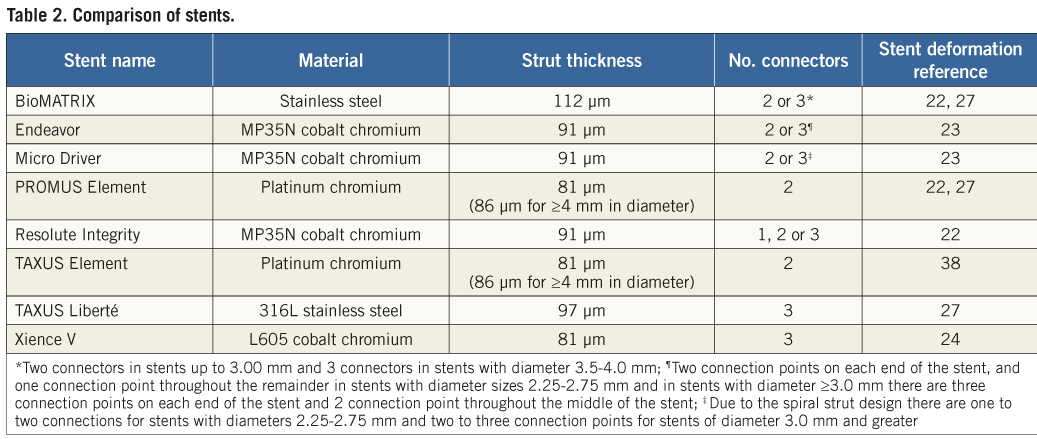
Ormiston and colleagues recently performed bench-top compression and elongation studies of different stent types. They concluded that a reduction in the number of connectors and strut thickness has successfully improved stent flexibility and deliverability with the trade-off of decreased longitudinal strength and the potential for longitudinal deformation25. Similarly, Prabhu et al used a standardised compressive force during bench testing of 14 commercialised coronary stent platforms from four stent design families and concluded that a specific stent design (offset peak-to-peak with two links connecting adjacent rings) plays a central role in determining longitudinal stent strength and the propensity for longitudinal compression19. Interestingly, these investigators found no apparent correlation between stent strut thickness and only a moderate correlation between the number of links/connectors between rings and the amount of longitudinal stent compression associated with a 50 gram force load19. However, the few estimates regarding comparative clinical frequency of this event in patients with different stent platforms are not definitive23,27. In this regard, with 2,403 stents examined, this report represents the largest systematic independent corelab analysis of longitudinal stent deformation involving three distinct DES platforms following deployment across the spectrum of stent lengths and target lesion complexity evaluated in this study. The major finding of this study is that the rate of longitudinal deformation does not appear to differ substantially between the stent platform types included in the present analysis. Specifically, there were no significant differences between the TAXUS Express2 and TAXUS Element stent platforms in the measured to nominal stent length ratios. There was a slight difference in stent length ratio between the PROMUS and PROMUS Element stent platforms of questionable clinical relevance. Of note, the ratio was slightly greater for PROMUS Element than PROMUS, arguing against differential longitudinal stent shortening with the PROMUS Element platform. Similar results were found when the analyses were restricted to only those patients who received post-dilatation in the PERSEUS and PLATINUM trials, in whom balloon-stent interaction with consequent stent shortening might have occurred at the time of re-crossing.
Study limitations
Although the ability to discern longitudinal distortion may be easier or more accurate with a more visible/radiopaque platform such as the Element stent (PtCr ION, PtCr PROMUS Element), no differential tendency toward longitudinal deformation was observed with the Element versus either the TAXUS Express2 or the Promus/Xience V stents. Although reassuring, this observation comes with several caveats. First, the present study represents a post hoc analysis of patients and target lesions which qualified for enrolment into two prospective, randomised clinical trials. Of note, the present study included only 513 ACC/AHA type C lesions (21% of all lesions), the type in which longitudinal stent deformation is most likely to occur. Further study of the relative frequency of longitudinal deformation of different stents is thus warranted in larger studies of highly complex lesions. Second, the angiographic corelab did not initially assess longitudinal stent deformation based on a pattern of dense or overlapping stent struts but instead utilised the surrogate of QCA measured length to nominal stent length. However, longitudinal stent deformation may occur without significant change in stent length and may be detectable only by intravascular ultrasound22. Third, given the different radiopacity levels between stent platforms, the present analysis based on angiographic strut density may have been inherently biased. Fourth, although the angiographic view angle might be expected to influence QCA measured stent length (foreshortening could result in a low ratio) the same angiographic technique was specified by protocol and analysis was performed by the same QCA corelab in both trials for all stent platforms included in analysis. Randomisation would be expected to balance out chance differences in foreshortening or suboptimal views between the devices. Fifth, the present study may have underestimated the true rate of longitudinal stent deformation, as minor degrees of stent lengthening or shortening might be “buried” in the angiographic cumulative frequency distribution curves. However, most published cases of longitudinal stent deformation have been dramatic, with estimated foreshortening ratios of ~0.6 or less,22-27 and such cases were excluded by core laboratory review of individual outliers. Thus, if minor degrees of longitudinal stent deformation did indeed occur, 1) the near-overlapping measured:nominal stent length cumulative frequency distribution curves suggests that its frequency of occurrence was similar with the different stent platforms in this study, and that 2) its clinical impact is minimal given the low rates of follow-up adverse events, especially with the PROMUS and PROMUS Element stents. Finally, it must be acknowledged that both investigative study protocols specified target lesion preparation by predilatation prior to study stent deployment and direct stenting was not allowed. Although high pressure post-dilatation of the stented segment with a non-compliant balloon catheter was performed frequently in both the PERSEUS (52.2% TAXUS Express; 52.7% ION) and PLATINUM (46.2% PROMUS Element; 51.3% PROMUS XIENCE) trials (a situation which has been associated with anecdotal cases of longitudinal stent deformation), there was infrequent utilisation of intravascular ultrasound, thrombus aspiration, embolic protection or other catheters which have also been incriminated in contributing to stent deformation21,30-33.
Avoidance and treatment of longitudinal stent deformation
Strategies to avoid longitudinal stent platform deformation which have been suggested include adequate target lesion predilatation/preparation, more optimal stent expansion with the stent delivery system, avoidance of wire bias, minimised guide catheter-stent contact during efforts to maximise guide-catheter support (particularly with ostial lesion locations), and use of finesse and not force if resistance upon re-crossing is met. It should be noted, however, that difficulty re-crossing a stent may not only be due to longitudinal stent deformation, but may also be caused by stent under-expansion, or stent struts protruding into the lumen after deployment, which is more common with thin stent struts and open cell designs. Anecdotal reports suggest a possible association between severe longitudinal stent deformation and the occurrence of stent thrombosis22,23,27. Thus, if longitudinal compression is strongly suspected, high pressure post-dilatation of the involved segment with a non-compliant balloon catheter should be performed with additional stent deployment as necessary to achieve an optimal mechanical result.
Conclusions
Longitudinal stent deformation is a recently recognised complication of coronary stent deployment. The present study suggests that this complication occurs with similar frequency in the different coronary stent types and target lesion complexities included in the analysis, and that severe longitudinal stent deformation is rare. The methodology described in this manuscript may be useful for future studies evaluating longitudinal stent deformation.
Acknowledgements
The authors thank Kristine Roy, PhD (Boston Scientific Corporation) for assistance in manuscript preparation and Scott Wehrenberg, MS and Ed McMullen, MMath (Boston Scientific Corporation) for statistical analysis.
Funding
Boston Scientific Corporation, Natick, MA, USA.
Conflict of interest statement
The authors wish to disclose the following conflicts of interest related to this manuscript:
D.J. Kereiakes received grant support/research contract from Boston Scientific, Cordis, Medtronic and Abbott Vascular and consultant fee/honoraria/speaker’s bureau from Boston Scientific and Abbott Vascular; J.J. Popma received Institutional Research Grants from Boston Scientific, Abbott Vascular and Medtronic, consultant for Boston Scientific, Abbott Vascular; L.A. Cannon received grant support/research contract from Boston Scientific, Abbott, Medtronic (modest); consultant fee/honoraria/speaker’s bureau from Boston Scientific, Abbott and Medtronic (modest); D.E. Kandzari received grant support/research contract from Abbott Vascular and consultant fee/honoraria/speaker’s bureau from Abbott Vascular, Medtronic, ev3, The Medicines Company; C.D. Kimmelstiel received honoraria/speaker’s bureau from Boston Scientific (modest) and Lilly, Daichi Sankyo (significant), research/clinical trials support from Boston Scientific; I.T. Meredith received research grant, consulting and speaker fees from Boston Scientific, Medtronic and Abbott; P.S. Teirstein has received research grants, speakers fees, and consultant fees from Abbott, Boston Scientific, and Medtronic; S. Verheye has no conflicts of interest to declare; D.J. Allocco and K.D. Dawkins are full-time employees and stockholders in Boston Scientific; G.W. Stone received consultant fee/honoraria/speaker’s bureau from Abbott Vascular, Boston Scientific and Medtronic.

