Abstract
Aims: Longitudinal stent deformation (LSD) is a recently described complication of PCI, but mechanisms contributing to its occurrence and associated clinical outcomes remain unclear. The FDA Manufacturer and User Facility Device Experience (MAUDE) database was searched to identify cases of LSD to gain insight into procedural and anatomical factors that predispose to this complication and associated clinical outcomes.
Methods and results: The MAUDE database is a voluntary international electronic reporting system whose aim is to capture major adverse events involving medical devices. Using defined search terms, we identified 57 unique cases of LSD ranging from 2004-2011. A significant increase in the reporting of LSD in the last two years was observed with most reported cases in stents based on the Element platform (90%). The lesions in which LSD was reported were complex (vessel calcification 26%; tortuosity 25%; long 28%; ostial disease 21%) and most frequently occurred following attempts to pass or withdraw secondary devices through a previously deployed stent (89% cases where mechanism identified). Adverse clinical outcomes including emergent cardiac surgery and acute and sub-acute stent thrombosis occurred in eight cases.
Conclusions: LSD can occur secondary to a variety of mechanisms; identification and treatment is important since adverse incidents such as emergent CABG and stent thrombosis may occur. A novel classification system is proposed to facilitate future reporting of this complication.
ntroduction
Stent platforms have significantly evolved over the past 30 years and the utilisation of thinner struts, use of newer alloys and innovative stent designs have allowed the manufacture of more flexible stent platforms that have enhanced deliverability in tortuous and heavily calcified vessels. Stent design is a balancing act of several attributes that contribute to stent performance such as crimped and deployed stent flexibility, trackability, scaffolding, radio-opacity, longitudinal and radial strength, and recoil1,2. Enhancing one particular stent attribute to improve deliverability may however adversely affect other stent attributes2. As an example of this phenomenon, Hanratty and Walsh described longitudinal stent deformation (LSD) as a “new’ complication associated with modern stent platforms in which shortening of a stent occurs in the longitudinal axis following initially successful stent deployment3. More recently, we have described a further nine cases of LSD identified over a four year period representing 0.2% of cases performed at our centre4. The occurrence of LSD is not benign: in our series there was one case of late stent thrombosis associated with LSD, whilst a recent case report has described subacute stent thrombosis of a deformed right coronary artery (RCA) stent5.
Recent engineering analyses have suggested that there are significant differences in longitudinal strength between contemporary stent platforms2,6 although the applicability of these observations to contemporary interventional practice remains unclear. Furthermore, whilst small case series derived from single centres serve to highlight this complication, it is difficult to draw firm conclusions on mechanisms and procedural and anatomical factors that predispose to this complication, as well as clinical outcomes, based on the 13 cases reported to date in the literature.
The U.S. Food and Drug Administration (FDA) Manufacturer and User Facility Device Experience (MAUDE) database is a voluntary international electronic reporting system whose aim is to capture major adverse events involving medical devices. We searched the MAUDE database to identify cases of LSD to gain further mechanistic insight into procedural and anatomical factors that predispose to this complication and associated clinical outcomes.
Methods
The FDA MAUDE database contains voluntary reports since June 1993 and manufacturer reports since August 1996. Data received are updated monthly and available free of charge to medical practitioners and the public. The on-line search function (http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.CFM) allows searches to be performed of the FDA Center for Devices and Radiological Health (CDRH) database for information on medical devices that may have malfunctioned or caused a death or serious injury. Each event report undergoes review by the FDA and the medical device company. The medical device company can respond to each event and their comment is incorporated into the original report and made publicly available as part of the database.
The database was searched from its inception in 1992 until 31st October 2011 using the following search terms; “accordion”, “accordian” (misspelt), “axial”, “compress”, “concertina”, “crumple”, “crumpled”, “shorten”, “shortened”, “longitudinal compression” and “longitudinal deformation” with the term “stent” added to each of the aforementioned search terms. Individual reports identified using this search strategy were independently studied by the two authors of this manuscript for the presence of longitudinal stent deformation defined as the distortion or shortening of a stent in the longitudinal axis following successful stent deployment. Records identified by the two authors as fulfilling these criteria were pooled, and duplicate records identified due to either multiple entries for a single adverse incident, or inclusion of multiple search terms were deleted. The remaining unique records were further analysed to identify procedural and anatomical data where available, potential mechanisms that contributed to the development of LSD, subsequent treatment and clinical outcomes. Figure 1 summarises the search strategy.
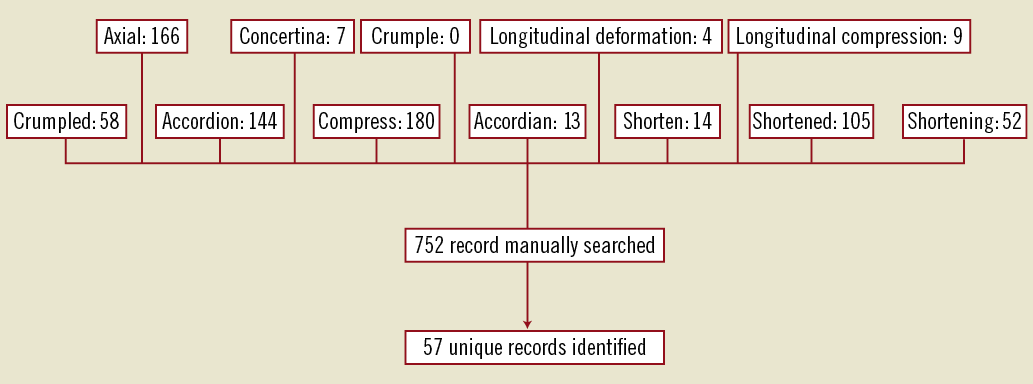
Figure 1. Search strategy used.
Results
A total of 752 records identified using our search strategy were manually searched in which 57 unique cases of longitudinal stent deformation were found. The years in which the FDA received reports of LSD ranged from 2004 until 2011, although the majority of the reports were derived from 2010 and 2011 (90%; Figure 2). Cases of LSD were experienced with ten different stents from six different platforms (Table 1). The most common stent platform affected of the reported cases was the Element platform (Boston Scientific, Natick, MA, USA) (45 cases out of 57; 79%) followed by the Driver platform (Medtronic Inc., Minneapolis, MN, USA) (5/57; 9%). There were three reported cases with the CYPHER stent (Cordis Corp, Miami Lakes, FL, USA) and one each with the TAXUS Liberté (Boston Scientific), XIENCE V (Abbott Vascular, Santa Clara, CA, USA) and Nobori (Terumo Corp, Tokyo, Japan).
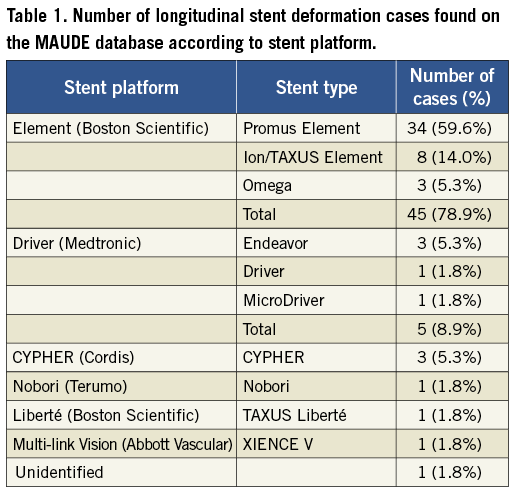
Figure 2. Number of cases of longitudinal stent deformation submitted to MAUDE website according to year.
Table 2 summarises the procedural characteristics of the reported cases. The vessel was not identified in three cases and lesion morphology was not described in five cases. The commonest vessel in which LSD occurred was the LAD (44% of cases in which the vessel was identified). The most common adverse vessel features associated with the occurrence of LSD were calcification (26%), tortuosity (25%) and a long lesion length (28%).
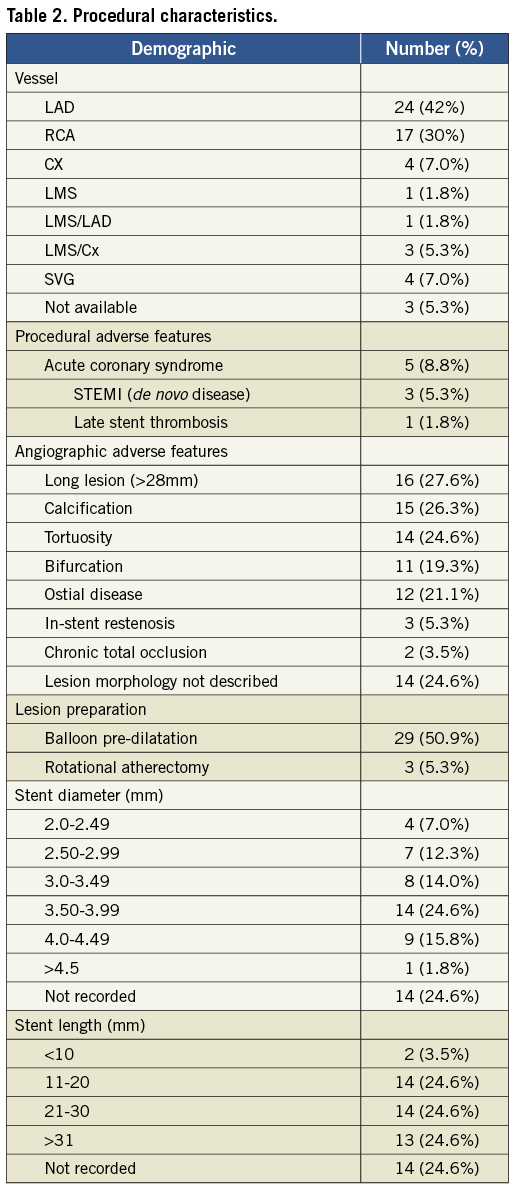
Table 3 shows the main mechanisms for LSD. Twelve reports did not have sufficient information to identify which end(s) of the stent were deformed. In those cases where the site of LSD was identified, 37 cases affected the proximal aspect of the stent (79%), whilst ten cases (21%) were deformed in the distal aspect. Two of the cases involved deformation at both stent edges. In one of these cases, a post-dilatation balloon could not be withdrawn from an Ion stent (Boston Scientific). Traction on the balloon caused distal stent deformation and deeply engaged the guide catheter causing proximal stent compression. It proved impossible to remove the balloon catheter from the highly deformed stent and the patient required emergent cardiac surgery to extract the trapped device (see Table 4, case 1). In the second case, following post-dilatation of a Promus Element stent (Boston Scientific) the stent rings at both ends were noted to be closer together. A further stent was deployed proximally without adverse clinical outcome.
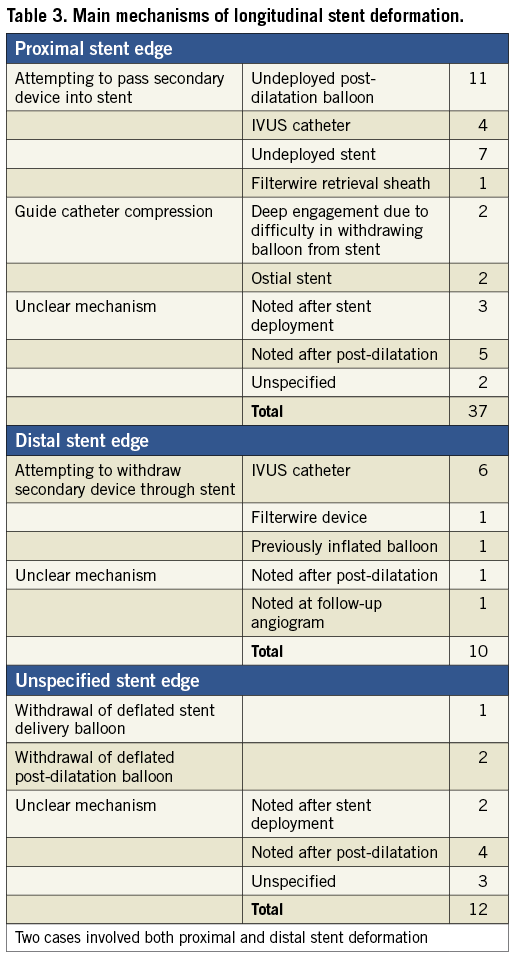
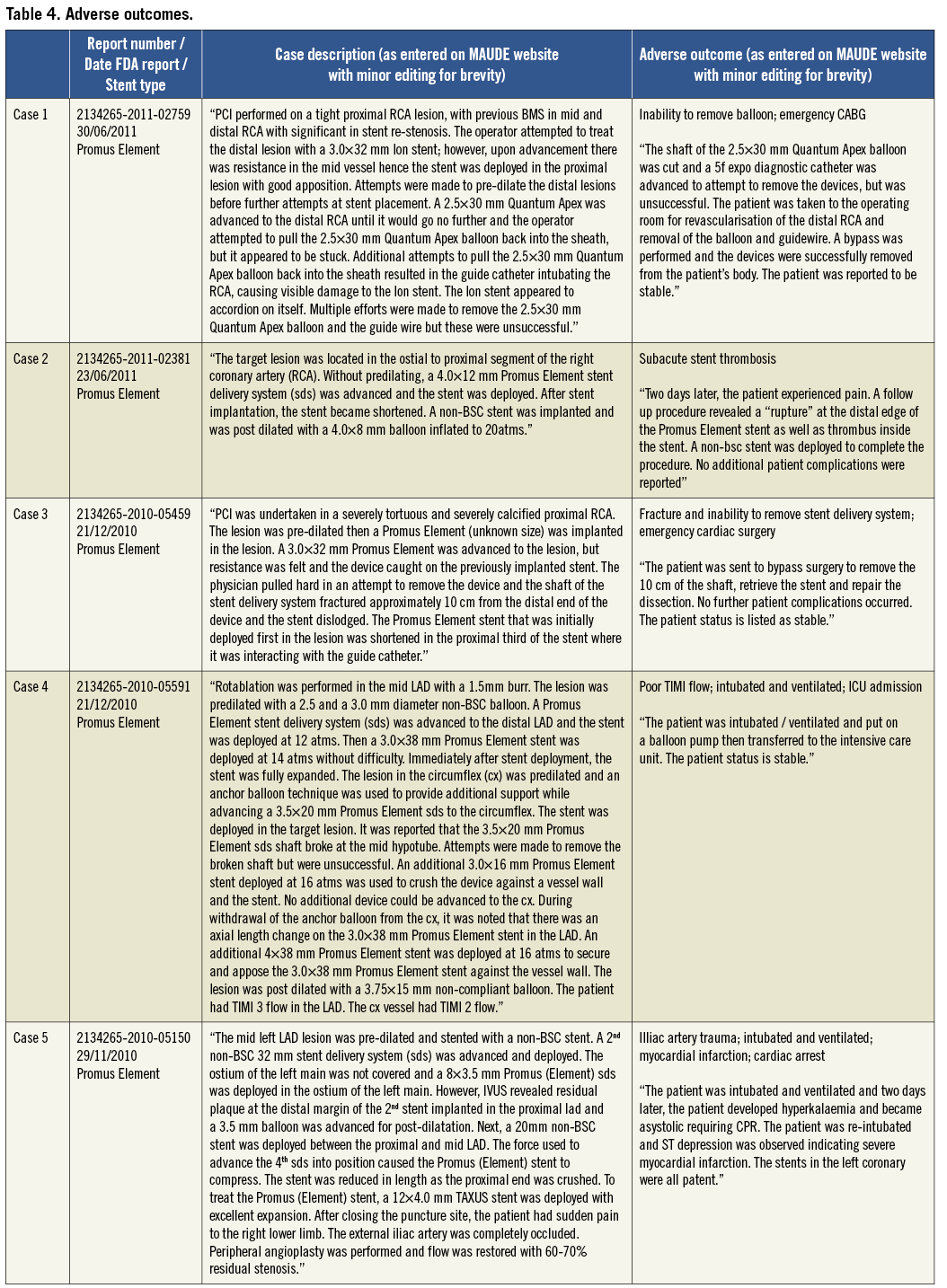
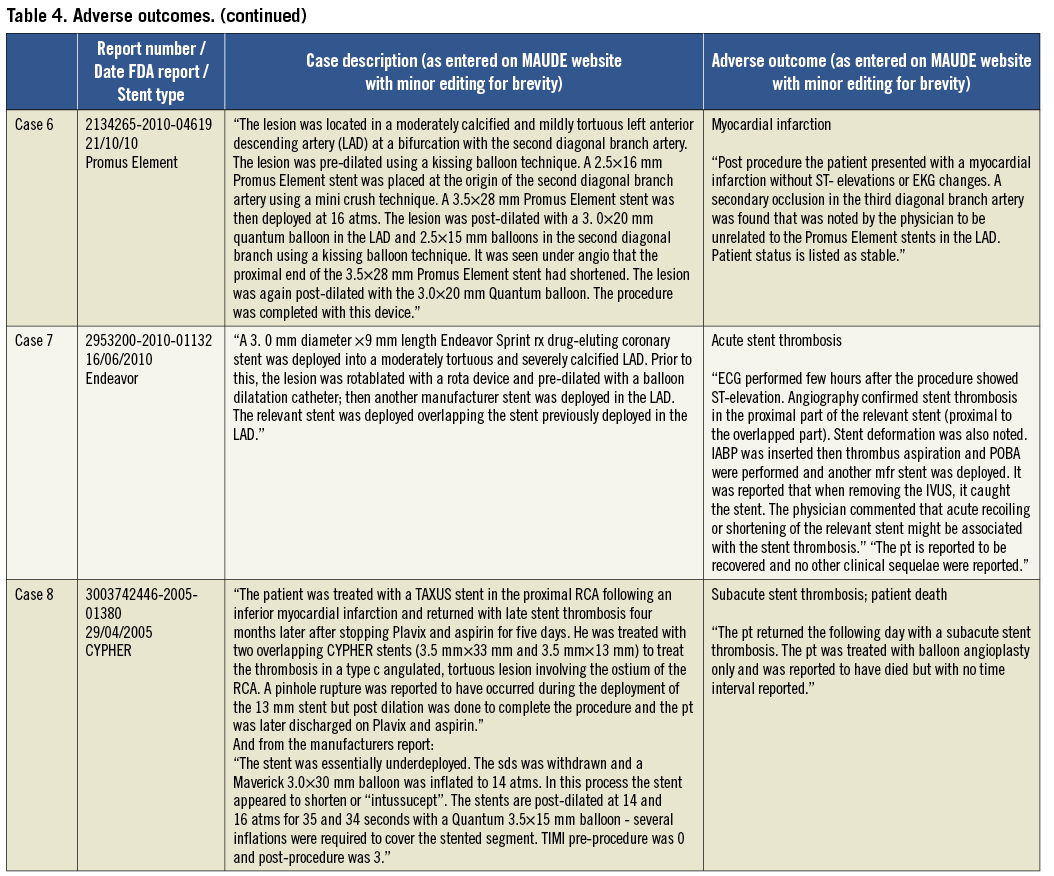
Out of the 27 cases of proximal stent deformation in which a mechanism could be identified, 85% were due to attempts to pass secondary equipment. This included an un-deployed post-dilatation balloon in the majority of cases (48%), a further stent (30%), an IVUS catheter (17%) and a Filterwire distal protection device retrieval sheath (4%). The other 15% of cases were due to guide catheter compression either of an ostial stent or due to deep guide engagement. All cases of distal stent deformation in which a mechanism was identified were due to attempts to withdraw secondary equipment, most commonly an IVUS catheter (75%).
The majority of LSD cases reported required further treatment (45/57 cases; 79%). Further stent deployment was performed in 33 cases (58%), and balloon post-dilatation was performed in ten cases (18%). Two cases required emergent cardiac surgery. Eight cases (14%) did not receive further treatment and treatment was not specified in the remaining four cases (7%).
Patient outcomes following the occurrence of LSD were reported in 56/57 cases (98%). In 49 of these cases there were no reported adverse outcomes (86%). The eight cases (14%) in which there was an adverse outcome are summarised in Table 4. Two patients underwent emergent cardiac surgery (3.5%) because of an inability to remove a device from a longitudinally shortened stent (one balloon in an Ion stent; one undeployed stent delivery system in a Promus Element stent). One acute stent thrombosis (Endeavor stent) and two subacute stent thromboses occurred (one CYPHER and one Promus Element stent). In one of these, patient death was reported but the timing was unclear. There was an acute coronary syndrome related to side branch occlusion at an unspecified time point following the index procedure which was not thought to be related to LSD (Promus Element stent). One patient sustained a cardiac arrest and myocardial infarction two days following a procedure in which LSD occurred, although there were other complications including iliac artery trauma which may have contributed and angiography revealed that the treated LSD stent remained patent (Promus Element stent).
Discussion
In the current analysis performed using a systematic search of the FDA MAUDE database using a defined search strategy, we have identified 57 unique cases of LSD and show that this is not a new complication, as has been recently suggested3, but one that was initially described as far back as 2004. The most common angiographic features found to be associated with the occurrence of LSD in the reported cases were long lesions, vessel calcification and tortuosity, and there was a high frequency of adverse procedural and lesion characteristics associated with the occurrence of this complication. In contrast to other studies, we show that guide catheter compression as a mechanism of LSD in ostially deployed stents is relatively uncommon (15%), and show that attempting to pass or withdraw secondary devices such as undeployed post-dilatation balloons, IVUS catheters, undeployed stents or distal protection devices through a previously deployed stent resulted in the majority of LSDs in this series where a mechanism was identified. LSD is shown to not always be a benign complication, but one that can be associated with significant morbidity and mortality, with adverse outcomes reported in 8/57 cases (14%). Finally, this analysis reveals that LSD is most frequently reported with the Element platform stents (45 out of 57 cases; 79%).
The primary purpose of this study was to examine mechanisms, treatments and outcomes for LSD. The cases in which LSD occurred were complex, with adverse procedural or angiographic features present in 89% of cases. Calcification was commonly reported, with several cases necessitating rotablation, which concurs with the finding in our recently published series, in which vessel calcification was present on angiographic review in 56% (5/9) cases.
The most common mechanism of stent deformation in reported cases was from attempts to pass a variety of secondary equipment either into (proximal LSD) or withdrawing back through (distal LSD) a stent, which together accounted for 89% of cases where a mechanism could be identified. Proximal LSD secondary to attempts to pass an undeployed post-dilatation balloon accounted for almost one quarter of cases with an identified mechanism. Angiographic review of cases of LSD caused by undeployed balloons at our centre has shown that the mechanism of proximal stent deformation is commonly related to wire bias on a vessel curve, often in an under-expanded stent4. However, the angiographic descriptions of this complication in the database reports were insufficiently detailed to confirm this mechanism.
Further treatment of cases in which LSD had occurred was reported in approximately three quarters of cases with the most common therapies being the use of a further stent (58%) or balloon post-dilatation alone (18%). In the current study one case of acute stent thrombosis occurred in which LSD at the index procedure had not been identified or treated. We and others have previously described cases of LSD that, if left untreated, are associated with stent thrombosis4,5. Nine cases of LSD were reported as not receiving any further treatment in this series and these patients may be at increased risk of future late stent thrombosis. Of further concern, there were two further cases of subacute stent thrombosis in this series that occurred despite recognition and treatment of LSD: one of these cases received a further stent and the other was treated with recurrent balloon dilatation at the time of the index procedure. This is the first time this has been reported and suggests that even with successful identification and appropriate treatment; LSD may be associated with a risk of subsequent stent thrombosis.
Worryingly, our analysis demonstrates that, even once identified, LSD may not always be possible to treat as there were two cases where LSD was diagnosed but equipment could not be withdrawn from the deformed stent: these patients needed emergent CABG to remove equipment and revascularise the vessel (both Promus Element stents). This serious complication of LSD has not been previously described in the literature. The case report of Robinson et al further demonstrates that treatment may not be possible even when recognised by experienced operators5. In this case wire position was lost and the deformed stent could not be rewired. The patient re-presented five days later with subacute stent thrombosis and subsequent intervention was unable to reopen the vessel resulting in a large myocardial infarction. This reinforces the importance of maintaining wire position within the deformed stent once this complication occurs.
It has been claimed that longitudinal stent compression is a “new” complication3, but this analysis shows that this complication has been reported to the FDA, albeit in small numbers, since 2004. However, there is a clear and substantial increase in reporting of this complication since 2010 (Figure 2). There are several possible reasons for this. Firstly, there may be an increase in reporting due to increased awareness of this complication amongst interventional cardiologists. Although this complication has gained widespread recognition in both interventional cardiology journals and the widespread media, and was discussed extensively at the Transcatheter Cardiovascular Therapeutics (TCT) conference in November 2011, we believe this is unlikely to be the sole explanation as the first reported case series of this complication in the literature was only published online on the 5th October 20113 and all cases identified in this series were reported to the MAUDE database before this date.
A second possibility is that there is a genuine increase in this complication in contemporary interventional practice, in which more complex disease is being treated that, in the past, may have been treated with surgical revascularisation or medical therapy. Whilst, contemporary interventional practice involves cases with many of the adverse features identified in our analysis associated with LSD, there has not been a sea change in interventional practice towards these types of cases in the past two years that would account for the majority of LSD that have been reported in a similar time frame.
Finally, it has been argued that LSD is a general problem common to all modern generation stents, which are typically constructed with thinner struts than previous generations to optimise deliverability and trackability, particularly in complex lesion types3. However, in 2010 and 2011, 45 of the 50 reported cases (90%) involved the Boston Scientific platinum chromium Element platform (Omega bare metal stent; Promus Element everolimus-coated stent; TAXUS Element/Ion paclitaxel-coated stent). Although MAUDE data is not intended to be used either to evaluate rates of adverse events or to compare adverse event occurrence rates across devices we believe this is a signal that the increased reporting of this complication is related to the introduction of the Element stent platform. This concurs with the finding of our recently published case series which reported an increased frequency of this complication with the Promus Element stent4.
Whilst the platinum chromium Element platform was developed to improve radiopacity, data from Boston Scientific reveals that the density and therefore the radiopacity of the platinum chromium Element platform (9.9 g/mm3; 81 µm struts) is comparable to other contemporary stent platforms such as the cobalt chromium XIENCE V platform (9.1 g/mm3; 81 µm struts)7. Furthermore, although stainless steel may have a lower radiopacity than modern generation alloys (8.0 g/mm3), stents constructed from stainless steel typically have much thicker struts (CYPHER: 140 µm; TAXUS Express: 131 µm) which would serve to enhance their radiopacity. It is therefore unlikely that radiopacity is the only explanation for the differences in reported incidence between the stent platforms.
Recently published bench testing experiments performed by Abbott Vascular engineers and by Ormiston and colleagues in New Zealand provide mechanistic explanations for the clinical finding of increased frequency of LSD with the Element platform2,6. Both of these studies showed that the Element stent platform possesses substantially less resistance to longitudinal compression than other widely used stent platforms. These studies also clearly show that strut thickness is not a primary determinant of longitudinal strength. As an example, the Vision platform (used in the Xience V drug-eluting stent) has significantly improved longitudinal integrity compared to the Element stent platform, despite having identical strut thickness (81 μm). It is likely therefore that the unique design of the Element stent, with an offset peak-to-peak design and only two connectors between each stent ring is responsible for its low longitudinal strength6.
Of interest, LSD was not identified in the published PLATINUM, PERSEUS Workhorse and PERSEUS Small Vessel trials of the Element stent series, in which outcomes in 2,034 patients using the Element Stent platform have been reported8-11. This may be because these trials involved relatively simple, de novo coronary lesions in which complex lesion characteristics such as moderate to severe calcification, severe tortuosity, bifurcation disease with a side branch diameter >2 mm, ostial disease, chronic total occlusion, recent myocardial infarction, use of rotablation, left main stem disease, vein graft disease, long lesions (>24 mm PLATINUM; >28 mm PERSEUS WH; >24 mm PERSEUS SV) and in-stent restenosis, were excluded8,12. The majority of cases of LSD in this analysis possessed at least one of these exclusion criteria (49/57; 86%), and would not have been eligible for inclusion within these trials.
Limitations
Whilst the MAUDE database is not intended to be used either to evaluate rates of adverse events or to compare adverse event occurrence rates across devices it contains the largest number of case reports in the world on adverse outcomes and malfunctions for medical devices and so provides an ideal opportunity to detect signals of new, rare and unusual adverse clinical outcomes such as LSD, which had a reported incidence of only 0.2% in the case series derived from our centre4. The detection of these signals has important implications for safety; the Council for International Organisations of Medical Sciences (CIOMS) defines a safety signal as: “A report or reports of an event with an unknown causal relationship to treatment that is recognised as worthy of further exploration and continued surveillance”13. Observational clinical studies and registries provide a framework for the detection of such signals, but their rigorous exclusion criteria exclude many of the adverse procedural characteristics that may predispose to the development of complications such as LSD. In contrast, the MAUDE database is more reflective of “real world” practice, when products are used for both on- and off-label indications, and so has an important role to play in the identification of such signals in real world contemporary PCI practice; it is with this purpose that this analysis was performed.
While spontaneous adverse event reports contained in the MAUDE database are very important for generating hypotheses and identifying safety signals, there are limitations. Firstly, since the reporting of adverse incidents to the MAUDE database is performed on a voluntary basis, it is likely that incidents of LSD are significantly under-reported. Furthermore, it is likely that there is an element of reporting bias in the submission of incidents to the MAUDE database whereby operators tend to report adverse incidents more often when they are accompanied by adverse outcomes and so this may strongly overestimate the causal relationship between LSD and unfavourable outcomes. Secondly, the MAUDE database does not distinguish between on-label and off-label uses of approved products. Thirdly, this analysis is a review of operators’ written reports, and therefore formal angiographic and case review, has not been performed and so is subject to interpretation bias. There was incomplete information regarding procedural and baseline angiographic characteristics in several of the reports, and in several cases it was not possible to ascertain the mechanism of deformation. Follow-up and patient outcomes will also be incomplete. By necessity, only database entries in which a user report has been submitted were reviewed. As this complication has only recently been formally described, operators have used varied terms to describe it that may not have been detected with our search strategy. Finally, although this data demonstrates a signal that this complication occurs with increased frequency with the Element platform, it cannot be used to definitively measure either the overall frequency of this complication or to compare the frequency between stent platforms.
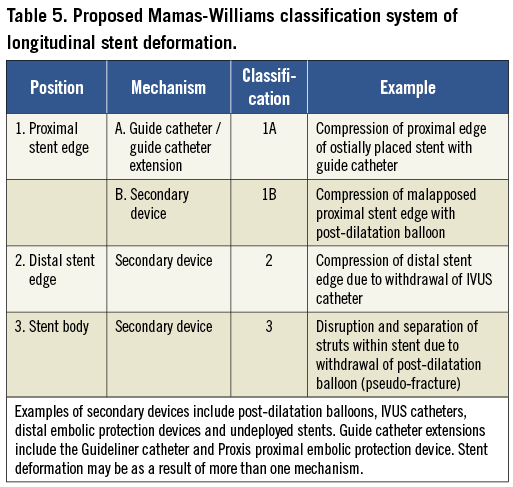
In conclusion, an analysis of the FDA MAUDE database shows that LSD has been reported since 2004, but that there has been a dramatic increase in reported cases in the last two years. LSD occurs most commonly in complex lesions, which are often excluded from clinical trials of new stent platforms. LSD is not always a benign complication with adverse incidents such as emergent CABG for retained equipment and acute and subacute stent thrombosis occurring, despite recognition and treatment in some cases. LSD was reported most commonly in Element platform stents and this signal warrants further analysis in a systematic manner with angiographic review of cases to examine mechanisms. There is an urgent need for future prospective studies using different stent platforms whose inclusion criteria are more reflective of real world interventional practice to define the incidence and outcomes of LSD. Based on the results of this analysis, and our previous angiographic review of cases at our centre4, we propose a novel classification system based upon the mechanism of LSD to standardise reporting of LSD in future studies (Table 5).
Finally, we would encourage all operators experiencing LSD to report their cases to the FDA MAUDE database to facilitate investigation into this complication.
Conflicts of interest statement
The authors have no conflicts of interest to declare.
Funding
This research received no specific funding.

