A 36-year-old dyslipidaemic, cigarette-smoking woman with a family history of CAD presenting with exertional angina had non-invasive evidence of inferior ischaemia. A severe proximal lesion of the RCA was treated as outlined in detail in Figure 1 and Figure 2 and their legends. A 4.0×20 mm Promus™ Element™ stent (Boston Scientific, Natick, MA, USA) was shortened due to the delivery balloon catching on the distal stent on withdrawal associated with deep guide catheter intubation. Stent shortening uncovered part of the lesion that was treated with an additional 4.0×8 mm stent of the same design. The post dilation non-compliant balloon pushed struts apart which appeared on CT angiography as stent discontinuity that could have been misinterpreted as strut fracture. This was associated with an intraluminal filling defect, presumably prolapsing plaque and thrombus. The disrupted stent was treated by implantation of a third stent (4.0×8.0 mm XIENCE PRIME; Abbott Vascular, Santa Clara, CA, USA) with a good angiographic result. The patient remains well at 8-month follow-up.
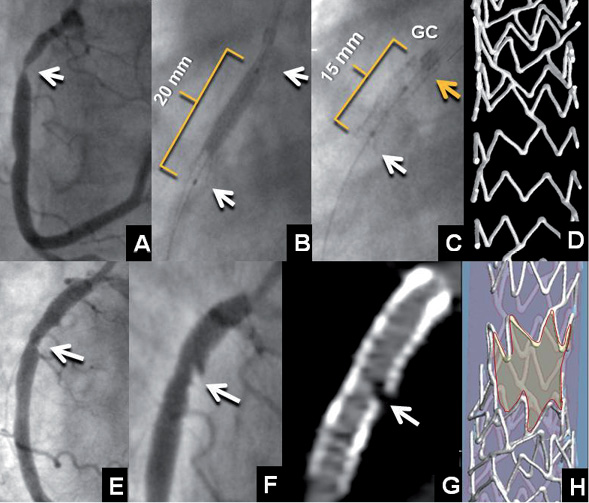
Figure 1. Baseline angiography (A) revealed a severe proximal RCA stenosis (arrow). At deployment (B), the Promus Element stent measured 20 mm in length and shortened by 5 mm after deployment (C). A likely explanation is that the winged deployment balloon caught the distal end of the stent (white arrow) pulling hoops together and simultaneously pulled the guide catheter (GC) onto the proximal stent end pushing hoops together. Note the increased radiopacity of the proximal end of the stent due to multiple strut crowding (yellow arrow). To assist in understanding these phenomena, frame D shows a micro-computed tomography of a Promus Element stent that has been shortened in a phantom by proximal hoops being pushed together by the guiding catheter. This image also shows the Element platform design (zigzag out-of-phase hoops with two angled connectors per hoop joining off-set peaks) conceived for flexibility and deliverability improvement. Frame E shows residual stenosis exposed by stent shortening. After deployment of an additional Promus Element stent and multiple post-dilatations with a non-compliant balloon, strut separation with thrombus and plaque prolapse was observed by invasive (F) and CT angiography (G). Frame H is a micro-computed tomography of a Promus Element stent that has been damaged in a mock artery by the distal tip of a non-compliant balloon pushing hoops apart and causing a pseudo-fracture.
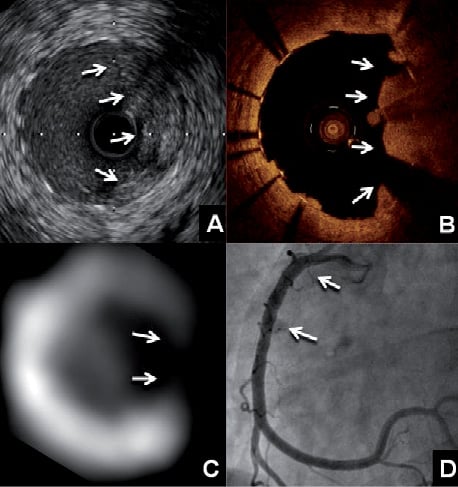
Figure 2. Intravascular ultrasound (A) showed plaque prolapse (arrows) at a region without stent struts from 12 o’clock to 6 o’clock. Optical coherence tomography (B) of the same region confirmed the 180° absence of stent struts, also demonstrated by the short-axis CT image (C), and a large thrombus adhering to the prolapsing plaque (arrows). Final angiography (D) showed a widely patent vessel (arrows) after implantation of another drug-eluting stent to the disrupted site.
Recently reported cases of stent longitudinal compression or elongation have been caused by a post dilation non-compliant balloon, a guide catheter or a device such as an intravascular ultrasound catheter1-3. While these phenomena have been observed with a number of stent designs1-3, they seem more common with the Element (Promus, Omega or Ion) platform2,3, which has been shown to have less longitudinal strength than other contemporary stent platforms4.
Our patient demonstrated stent longitudinal distortion with stent compression and strut separation. Additional stenting was required to treat a stenosis that was uncovered by stent shortening, then further stenting was needed for strut separation that had caused plaque prolapse and thrombus formation. Stent design involves trade-offs, and improvement in flexibility and deliverability achieved by modifying connector numbers and configuration can be at the expense of longitudinal integrity4.
Conflict of interest statement
A. Bartorelli, Advisory Board and minor honoraria for Abbott Vascular; J. Ormiston, Advisory Board and minor honoraria for Abbott Vascular and Boston Scientific. The other authors have no conflicts of interest to declare.

