Introduction
Intracoronary bare metal stent (BMS) platforms have evolved significantly over the last three decades. Initially, the vast majority of stents were manufactured from stainless steel and varied somewhat in their basic manufacture, cell geometry and strut thickness.1 Manufacturers focused on many areas of development in order to produce stents that were both user friendly and that improved clinical outcomes. As the technology improved, so did the practice of percutaneous coronary intervention (PCI).
Ultimately, in-stent restenosis (ISR) was accepted to be a function of several factors: smaller diameter stents, longer stents and the presence of diabetes increased ISR. The advent of drug-eluting stents (DES) has since significantly reduced the risk of ISR. The implications of this positive technological advance have been threefold. Firstly, the practice of interventional cardiology has been transformed. Cardiologists no longer aim to treat focal areas of the most severe coronary narrowing, but now aim to treat arteries from “normal to normal” segments, where there is no significant atheroma present at either stent edge. Therefore, longer stents are much more commonly required and are now generally used preferentially. Secondly, as clinical evidence has improved2 more challenging lesions are treated as normal, often in the setting of multivessel disease. DES have facilitated PCI for patients with extremely challenging coronary lesions where cardiac surgery is not an option due to comorbid factors. Thirdly, manufacturers have responded to feedback from clinicians and the consequent “market forces” that have accompanied these changes in clinical practice. The effect has been concomitant development towards longer, thinner, more flexible stent platforms that are much less difficult to deliver even in the most difficult of vessels with extreme tortuosity and calcification (Table 1). Therefore, PCI for disease that would not have been treatable 15 years ago is now commonplace.
Pragmatically, mechanical engineering is a science of compromise. Altering any one feature of a stent platform will modify other aspects of how a stent performs. Whilst thinner struts and a lower metal:artery ratio aid stent delivery, the downside is to reduce radial strength. Reducing the number of fixed connectors between cells and altering the geometry of these connectors and their longitudinal distribution enhances flexibility and conformability, but at the expense of the longitudinal strength of the stent structure. The longitudinal strength or compressibility of coronary stents has never been reported as a standard parameter nor previously considered important. We describe a case series detailing that stent longitudinal strength is a very relevant and important feature of these devices, especially in the contemporary practice of interventional cardiology.
Case series
Case 1
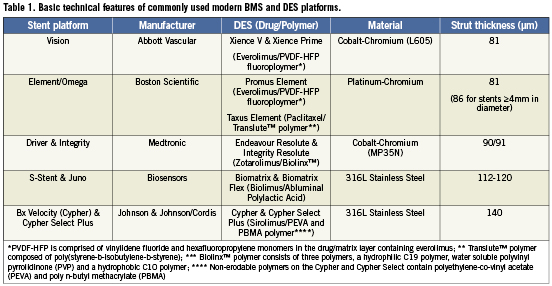
A 50-year-old male was admitted for PCI. There was a history of longstanding type 1 diabetes and 3-vessel coronary artery bypass grafting (CABG) eight years previously. The patient had re-presented with CCS class 3 angina and progression of both native coronary artery disease and graft disease. Further to reconstruction of his native left anterior descending coronary artery (LAD), he returned for a staged PCI to reconstruct his native right coronary artery (RCA). There was critical disease of the saphenous vein graft to the RCA. The native vessel demonstrated diffuse disease throughout the main RCA with a functional chronic total occlusion at the origin of the posterior descending branch. After crossing the occlusion and predilating the vessel, a total of four Promus Element Platinum Chromium DES (Boston Scientific, Natick, MA, USA) were deployed in the vessel. A single 2.25/16 mm stent was deployed in the mid posterior descending branch. Then three overlapping 3.0/38 mm Promus Element stents were deployed from just beyond the crux of the RCA, back to the ostium (Figure 1, Panels A and B). At this point, a satisfactory ostial stent result was obtained. The stented segment was then postdilated in keeping with our routine practice. In order to deliver a 3.5 mm non-compliant balloon to the distal third of the RCA, guide manipulation was required to provide better support. After manoeuvrings the guide, it became apparent that the ostial segment of the original most proximal Promus Element was compressed longitudinally as a result of the guide catheter manipulation (Figure 1, Panel C). In this case, the enhanced radiographic visibility of this platform meant that the complication was immediately apparent. This compressed proximal stent required further postdilation to facilitate deployment of another stent to cover the ostial segment of the RCA. A final 3.5/12 mm Promus Element was deployed at this ostial segment and postdilated. Ultimately, a technically satisfactory result was obtained with no adverse consequences for the patient who remains well at follow-up (Figure 1, Panel D).
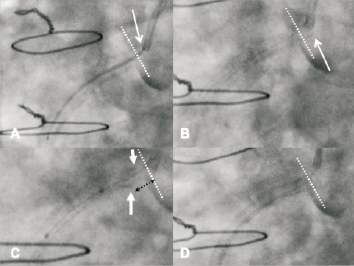
Figure 1. Panel A: Following vessel reconstruction a 3.0/38 mm Promus Element is deployed with the proximal edge (arrow) in the aorta proximal to the plane of the RCA ostium (dotted line). A contained and limited dissection is present in the sinus of Valsalva in all panels. Panel B: Proximal edge of the stent is seen covering the RCA ostium and sits just into the aorta (arrow). Panel C: Following guide catheter manipulation to facilitate distal vessel post-dilation, the most proximal stent has shortened. The proximal edge no longer covers the RCA ostium (dotted white line). The area of shortening is demarcated by the dotted black line. A dense radio-opaque segment is visible at the compressed proximal stent edge (paired white arrows). Panel D: Final picture following further stent deployment covering the RCA ostium.
In this particular case, the Promus Element stent at the ostium of the vessel was crushed longitudinally as an Amplatz 1 guide catheter was advanced into the vessel to provide improved guide support. We did not feel that this issue was related to the stent platform itself, as a braided guide catheter is capable of deforming any coronary stent. The decision to deploy a further Promus Element rather than an alternative platform was made to keep the drug used and delivery within the coronary as consistent as possible. This decision was vindicated by the technically satisfactory result that was achieved.
Case 2
A 72-year-old male presented with an acute coronary syndrome. Angiography demonstrated focal disease in the RCA and distal left main stem (LMS) disease extending to the mid LAD. The proximal left circumflex was not involved. CABG was considered, but the patient refused as he was the sole carer for a dependent family member. PCI was performed with Biomatrix DES (Biosensors Interventional Technologies, Singapore). Treatment of the RCA was unremarkable. The treatment strategy for the distal LMS disease was deployment of a single stent into the LAD, with “provisional treatment” of the left circumflex (LCx). Two overlapping stents were successfully deployed from the mid LAD back to the ostium of the LMS (3.5/18 distally, 3.5/28 proximally. Postdilation performed with a 3.5 mm non-compliant balloon in the LAD and a 5 mm non-compliant balloon in the LMS). After postdilation, a routine intravascular ultrasound (IVUS) assessment was performed, with the full IVUS run being captured without complication. The images from the initial IVUS assessment were satisfactory (Figure 2). However, during removal of the IVUS catheter from the stented segment, this device snagged in the mid LAD, and resistance was felt on withdrawal of the imaging catheter. Ultimately, relatively strong force was required to remove the IVUS catheter from the vessel. Whilst the imaging catheter was stuck within the mid vessel stent, withdrawal also pulled the guide catheter into the stent that had already been deployed and postdilated at the LMS ostium. It then became apparent that the proximal stent was compressed longitudinally at both the proximal and distal edges. After the problem had been diagnosed, multiple postdilation balloon inflations and the deployment of one further stent in the mid LAD (3.5/14 Biomatrix) led to a satisfactory technical outcome. Again, the patient suffered no adverse sequelae and remains well at follow-up.
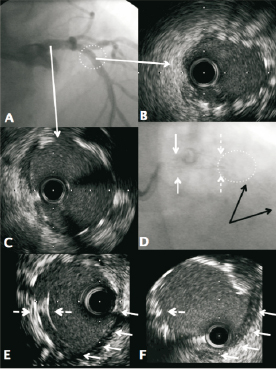
Figure 2. Panel A: Angiographic appearance following stent deployment from the mid LAD back to the LMS ostium using overlapping Biomatrix stents, (3.5/18 and 3.5/28 mm) There was only a single stent deployed in the LMS. The stents were optimised with non-compliant balloons. The area of stent overlap in the proximal LAD is highlighted (circle). Panel B: Corresponding IVUS of the overlapped area in the proximal LAD showing well apposed stents. Panel C: Corresponding IVUS of proximal LMS showing good stent sizing and apposition circumferentially. Panel D: Angiographic appearance after difficulty removing the IVUS catheter. The IVUS catheter snagged in the proximal LAD whilst the guide catheter was pulled forwards into the LMS. The distal edge of the more proximal stent now appears disrupted (composite shadow at dotted arrows) and there is no longer stent overlap in the mid LAD (circle). In addition the ostial LMS component of the same stent is disrupted (subtle composite shadow, solid white arrows). The distal LAD stent is demarcated (black arrows). Panel E: IVUS of the same proximal LMS region as Panel C, showing a double layer of longitudinally compressed stent (dashed arrows) and an area without stent coverage (grouped arrows) on the lower surface of the vessel. The stent was shortened in an eccentric fashion that was more exaggerated on the lower surface of the vessel. Panel F: Final IVUS of the same proximal LMS region following further dilation with non-compliant balloons. The “double layer” of stent has been corrected (dashed arrow) whilst the area without stent coverage is still present (grouped arrows).
Case 3
A 78-year-old male patient presented with unstable angina. Angiography demonstrated severe multivessel disease with involvement of the distal LMS. The patient was not suitable for CABG on the basis of multi-system comorbidity and PCI was performed. During the PCI procedure, routine angiographic imaging demonstrated a satisfactory stent outcome with Resolute Integrity DES (Medtronic, Minneapolis, MN, USA). There was no suspicion of any stent compression radiographically. However, IVUS assessment demonstrated that the stent had been compressed in a concertina fashion by the guide catheter at the ostium of the left main stem (Figure 3). This stent compression had occurred when the guide catheter was used to re-engage the LMS after this vessel had already been postdilated (5 mm non-compliant balloon). This abnormality was resolved with further balloon postdilation and a technically acceptable result was obtained at the LMS ostium with no need to deploy a second stent. Once again, there were no adverse consequences for the patient.
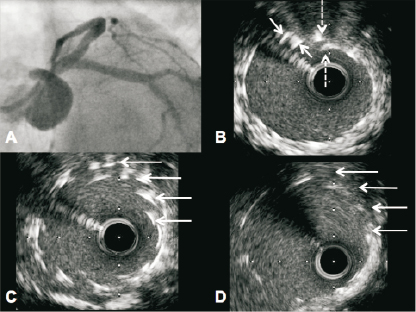
Figure 3. Panel A: Angiographic result following bifurcation stenting of the LMS, LAD and LCx. The LCx was treated by “mini-crush” with minimal stent protrusion (~1 mm) into the LMS. A 3.5 x 38 mm Resolute Integrity was deployed from the mid LAD to the LMS ostium and post dilated to 4.5 mm in the LMS. A kissing inflation was performed at the bifurcation with an excellent outcome at the carina of the LAD and LCx on IVUS. Respiratory motion of the guiding catheter was apparent throughout the case (sheathless 7 Fr JL 3.5, Asahi Intecc, Aichi, Japan). Panel B: IVUS of the proximal LMS demonstrating double layers of stent (arrows). Panel C: IVUS of ostial portion showing multiple layers of stent (arrows) in keeping with proximal stent concertina. Panel D: IVUS of the same ostial region following further post-dilation with a 5.5 mm balloon. This has resolved the proximal stent compression (arrows).
Discussion
In each case, the disease complexity mandated treatment of difficult coronary lesions including aorto-ostial disease. Such procedures are now part of the routine daily practice of PCI for appropriately experienced operators. During these and similar PCI procedures in an effort to optimise the mechanical, technical and thus long-term clinical outcome multiple and often complex steps are routinely performed. This may include the use of extra support guide catheters, aggressive guide catheter manipulation (deep intubation with guide advancement well into the target vessel), mother and daughter catheter systems, multiple balloon postdilations (including simultaneous kissing inflations), bifurcation stent techniques, rotational atherectomy and/or adjunctive imaging (IVUS or optical coherence tomography). Each manoeuvre may increase the potential for longitudinal stent compression.
In the described cases, compression of a previously delivered stent along its longitudinal axis occurred. This led to a marked alteration of the initial stent result that if unrecognised during the initial PCI, would have resulted in a grossly suboptimal technical result for the medium to long-term. It is our belief that this would have left a nidus for stent thrombosis and a potentially catastrophic late complication. In addition, a previously injured segment of vessel would have been left uncovered by any scaffold as well as untreated by local drug delivery with an added latent risk of restenosis. It is worth noting; that this complication may be angiographically obvious (Case 1), subtle (Case 2) or only be noted on adjunctive imaging (Case 3).
This complication of PCI is very rare. In our centre, we perform over 2,000 PCI cases per annum. Approximately 15-20% of cases involve rotational atherectomy, chronic total occlusion or left main intervention. The abnormality described, has been observed by high volume operators (>300 PCI cases per year, per operator) who regularly perform a large number of complex PCIs and take appropriate care during the treatment of aorto-ostial disease. We have observed only a handful of similar cases. We first noticed this complication with the Promus Element platform, where it was immediately apparent with due to the enhanced radiographic visibility of the stent. However, we have since observed this phenomenon within our institution with all modern DES platforms from each of the major manufacturers.
Therefore, we suggest that clinicians consider this issue. Care should be taken during PCI for ostial lesions. Deep intubation with guide catheters or extension systems should be performed with due care through already stented segments. Specific manoeuvres can be considered to avoid guide catheters moving in and out of the proximal coronary segments with respiratory or cardiac motion (such as leaving an additional guidewire in the sinus of Valsalva). Caution is also required after a long stent is deliberately relatively under-expanded in a larger diameter segment of the proximal vessel. If the more proximal stent edge lies within a tortuous segment, there is also a small potential risk that advancing further equipment through this area can catch the deployed stent before this area has been optimised.
A similar issue has recently been observed with postdilation balloons and the Endeavour and Micro Driver platforms.3
When a stent has been deployed and there is resistance to the delivery of postdilation balloons or imaging catheters, suspicion should be aroused. Very careful radiographic assessment of the stented segment should occur including comparison of the areas definitely covered by stent immediately post-deployment versus when the mechanical issue becomes apparent. Where possible, IVUS or optical coherence tomography should be used to confirm the diagnosis of longitudinal stent compression. Thereafter, postdilation of the compressed segment and further stent deployment should be performed as needed in order to produce an optimal mechanical result.
Finally, we suggest that stent manufacturers also consider this issue in future technological developments. Perhaps reporting longitudinal stent strength or compressibility should also be viewed as a standard piece of information in addition to the usual data that already come with the product brochure? Adopting an agreed industry standard to assess this parameter would also be a helpful advance.
Conflict of interest statement
The authors have no conflicts of interest to declare.

