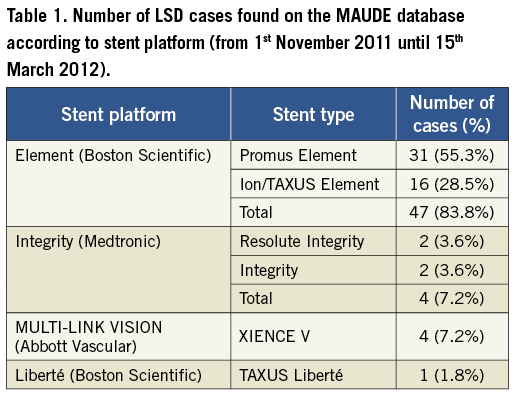
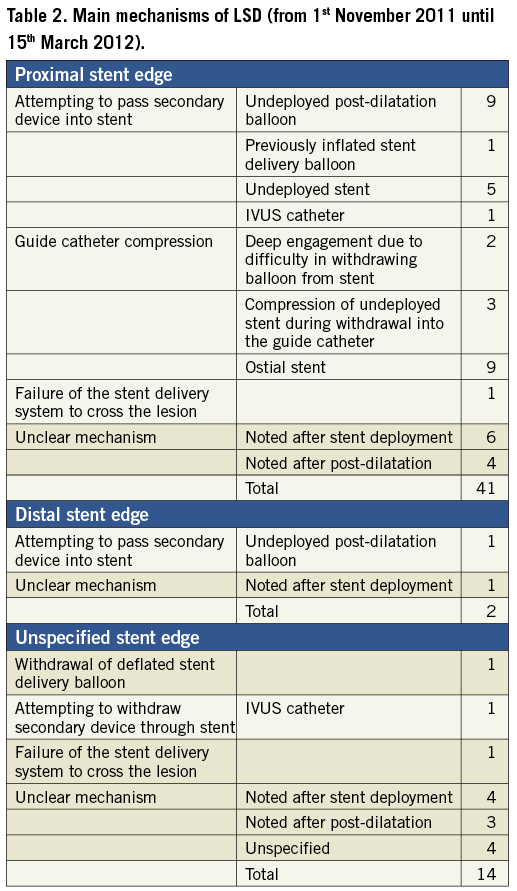
We read with interest the article of Mamas et al which reports on mechanisms and clinical outcomes of longitudinal stent deformation (LSD) taken from the FDA MAUDE database beginning with its creation in 1992 until 31st October 20111. In order to shed further light on this clinical issue, we have extended the search from 1st November 2011 until 15th March 2012 using the same search terms and the same methodology as described in the article. Individual reports were independently studied by the two authors of this letter, including careful analysis of stent types, causal mechanisms and clinical outcomes. Duplicate records were deleted. Out of this 20-week period search, we identified 56 additional cases of LSD (Table 1). Again, the most common stent platform involved was the Element platform (Boston Scientific, Natick, MA, USA) with 48 cases out of 56; 86%, followed in equal number by the Integrity platform (Medtronic Inc., Minneapolis, MN, USA), 4/56; 4% and the MULTI-LINK platform (Abbott Vascular, Santa Clara, CA, USA). There was one reported case with the Liberté platform (Boston Scientific). Main mechanisms of LSD are summarised in Table 2. Among the 43 cases that reported the site of deformation, 41 involved the proximal stent edge (95%) and two the distal stent edge (5%). One case involved deformation at both stent edges. The primary mechanisms of proximal stent deformation were attempts to pass secondary equipment (16/41; 39%) and guide catheter compression (14/41; 34%). Of note, we have identified five cases (9%) of LSD before any stent deployment: three cases (including two cases from our centre)2 involving major proximal stent deformation during withdrawal of the balloon-stent system into the guide catheter, and due to guide catheter crushing of the proximal stent edge, and two cases with significant deformation of an undeployed stent while attempting to cross the index lesion. In those cases involving the Element stent platform the diagnosis was made by fluoroscopy in all cases except one which was made by IVUS. Concerning the other platforms, the diagnosis was made by IVUS in three out of the four cases involving the Integrity platform (75%) and in two out of the four cases involving the MULTI-LINK platform (50%). Patient outcomes were reported in 55/56 cases (98%). There were four reported cases with adverse outcomes (7%). A myocardial infarction (MI) related to acute stent thrombosis occurred in three patients, and another MI due to subacute stent thrombosis in one patient. Out of these four cases, one patient required emergent CABG and one patient ultimately died. From this additional 20-week search of the FDA MAUDE database we can draw the following conclusions: 1) although LSD can occur with various stent platforms, we confirm that the Element platform accounts for the vast majority of cases included in this database, which could be partially related to the increased radio-opacity of this specific stent platform; 2) LSD can actually occur before any stent deployment and we suggest that the definition proposed by Mamas et al should be modified accordingly; 3) LSD can lead to adverse outcomes as serious as stent thrombosis, emergent CABG and death. We would urge operators to prospectively collect and report all cases of LSD in order to assess the exact incidence, the outcome and the real impact of this complication on our clinical practice.
The follow-up analysis performed by Aminian and Lalmand of the FDA MAUDE database to study the mechanisms and clinical outcomes associated with longitudinal stent deformation (LSD) using the methodology described in our original article is both complementary to our initial study and validates many of our findings. This analysis substantially increases the number of reported LSD cases to the FDA MAUDE database (as of March 2012) and as such represents the largest such collection of cases reported in the literature to date and offers an important opportunity to study an infrequent but important complication (with a reported incidence of around 0.2%1) in sufficient numbers to be informative.
Aminian and Lalmand identified five cases of deformation to undeployed stents following attempts to cross a lesion or during withdrawal of the stent delivery system back into the guide catheter. We also identified several cases of deformation to undeployed stents during our search of the MAUDE database but did not include these in our analysis as they did not fulfil the definition of LSD as: deformation of a stent due to force applied in the longitudinal axis following “initially successful stent deployment”1,2. Other factors such as balloon flexibility, strut thickness and crimped profile may be more important than longitudinal stent integrity with regards to the risk of damage to undeployed stents. We therefore do not believe these cases should be classified as LSD and would respectfully disagree that this mechanism ought to be included in our classification system2.
Despite this caveat, a further 51 cases (discounting the five cases of undeployed stent deformation) have now been identified over the four month period since the initial description of LSD by Hanratty and Walsh in November 20113, which is almost the same number of cases (57) identified over a period of seven years in our initial analysis2. Worryingly, this suggests that this complication may have been significantly under-reported previously, and is more common than previously thought. The current findings add to our initial observations that LSD is associated predominantly with the Element platform, affects mainly the proximal edge of the stent, is mainly due to secondary equipment delivery and is not a benign complication, associated with serious adverse outcomes including emergent CABG, stent thrombosis and death.
Finally, in the current analysis, 98% of cases (47 out of 48 cases) of LSD involving the Element stent platform were identified by fluoroscopy whilst 62.5% of cases (five out of eight cases) involving other platforms were identified using IVUS, although the clinical settings of these cases and the degree of LSD for each individual case is unclear. The authors have interpreted this finding as that the increased incidence of LSD associated with the Element platform may be partially related to the increased radio-opacity of this specific stent platform. Although the increased radio-opacity of the Element platform has been used to explain the higher incidence of LSD reported with it, we believe it is extremely unlikely that this is the only explanation. Firstly, LSD has only rarely been reported with other radio-opaque stents such as the first generation Cypher drug-eluting stent. More importantly, many reported cases of LSD have been noticed due to problems re-crossing an already deployed stent, i.e., they are diagnosed because of a procedural issue rather than the angiographic appearance alone. IVUS is a very sensitive modality for diagnosing LSD and the finding that LSD was more frequently diagnosed with fluoroscopy than with IVUS in Element stents, as compared to other platforms, may simply reflect that more minor degrees of stent deformation were occurring with non-Element stents. In fact, in many cases of stent deformation described in the MAUDE database, and experienced in our centre, it would have been extremely difficult to pass an IVUS catheter following LSD due to significant stent deformation. Finally, engineering analysis has suggested that the offset peak-to-peak, 2-connector design of the Element platform predisposes susceptibility to LSD in the deployed state4,5.
Stent design is a balancing act of several attributes that contribute to stent performance such as crimped and deployed stent flexibility, trackability and longitudinal and radial strength. Enhancing one particular stent attribute to improve deliverability may adversely affect other stent attributes hence interventional operators should develop a greater understanding of the design and limitations associated with each individual platform and use the individual platforms according to which design parameters are thought to be more important for a particular lesion / vessel anatomy / procedural situation. Analysis of the FDA MAUDE database by Aminian and Lalmand and ourselves provides further mechanistic insight into LSD and provides important information relating to procedural factors and lesion anatomy where this complication is more likely to be encountered; consideration of the engineering properties of stent platforms to be used in these type of cases may help to minimise the occurrence of this complication.

