Abstract
Aims: Stent fracture is important because it may cause adverse events. The interventional cardiologist needs independent data to aid stent selection. Stent designers need data to improve stent design. We used a repetitive bend test to compare durability and fracture for different stent designs.
Methods and results: Tested were 15 examples of six designs (BioMatrix Flex, Vision, MULTI-LINK 8, Element, Promus PREMIER and Integrity). One end of a nominally deployed stent was mounted on a fixed mandrel. The other end was translated a distance of ±3.5 mm at a rate of 6 Hz until fracture or 10 million cycles completed. The numbers of cycles to fracture for the Vision design (288,411±193,391) and the MULTI-LINK 8 (314,475±239,869) were significantly greater than for the BioMatrix Flex design (38,904±13,160), p<0.001. The difference between Vision and MULTI-LINK 8 was not significant (p=0.79). The Element, PREMIER, and Integrity designs did not fracture. Most fractures were in the curved portions of connectors between hoops.
Conclusions: The stent design which fractured most readily was the BioMatrix Flex. The most flexible designs did not fracture and, in general, stents with three connectors were more likely to fracture than those with two connectors between loops.
Introduction
While drug-eluting stents (DES) have transformed percutaneous coronary intervention, they continue to be associated with ongoing late clinical events1 such as stent thrombosis, target lesion revascularisation and even death. These events may be related to increasing intimal hyperplasia, late restenosis, and neoatherosclerosis that may be caused by stent strut fracture2-6. Recognised for at least 10 years7, stent strut fracture in early reports was most common in the CYPHER stent (Cordis, Miami, FL, USA)2,8,9. More recently, there have been reports of strut fracture in second-generation DES including XIENCE, XIENCE PRIME (Abbott Vascular, Santa Clara, CA, USA) and the Element design (Boston Scientific, Natick, MA, USA)5,6,10. Lesion and procedural factors such as arterial flexion, heavy calcification, stent overlap, stent length, right coronary artery location and implant duration may all predispose to strut fracture. In addition, different stent designs have different susceptibility to fracture. Independent data may aid device selection11 and aid development of improved devices12.
The aim of this study was to compare susceptibility to fracture for different contemporary DES designs using a repetitive bend accelerated fatigue to fracture test method.
Methods
The six stent designs tested (Figure 1) were the Vision (Abbott Vascular, Santa Clara, CA, USA), MULTI-LINK 8 (Abbott), BioMatrix™ Flex BES (Biosensors International, Singapore), Integrity (Medtronic, Santa Rosa, CA, USA), OMEGA™ or Element (Boston Scientific, Natick, MA, USA) and the Promus PREMIER™ (Boston Scientific). The drug-eluting version of Vision is XIENCE V, of MULTI-LINK 8 is XIENCE PRIME and XIENCE Xpedition, of Integrity is Resolute Integrity and of OMEGA is PROMUS Element, PROMUS Element Plus and TAXUS Element (called ION™ in the USA). Fifteen 3.0 mm diameter examples of each design were tested. Drug coating does not alter the potential for fracture of a stent platform on the bench although clinically DES are associated with more fractures than bare metal stents13-16.
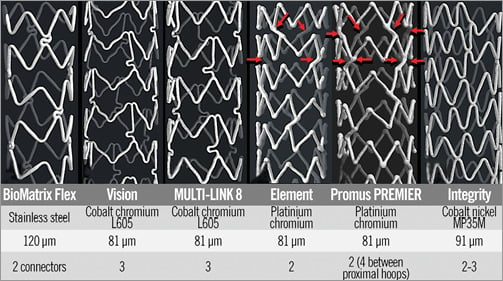
Figure 1. MicroCT images of the stent designs tested, construction metal, strut thickness and the number of connectors between hoops. The BioMatrix Flex, cut from a stainless steel tube has a strut thickness of 120 microns. It is designed with out-of-phase sinusoidal hoops with peaks linked by two “S” shaped connectors. The Vision and MULTI-LINK 8 and their drug-eluting XIENCE counterparts are cut from cobalt-chromium tubes with struts 81 microns thick. It has in-phase sinusoidal hoops with peaks and valleys linked by three bridges that are aligned with the stent long axis. Each connector has a “U” shaped loop to improve flexibility. The Element has bare metal and drug-eluting (everolimus and paclitaxel) versions. It is constructed from platinum-chromium, the strut thickness is 81 microns, and it is designed with sinusoidal hoops with off-set peaks linked by two straight bridges per hoop. The Promus PREMIER design is the same as the Element except that there are additional connectors between the proximal three hoops (red arrows). The design of the Integrity and its Resolute drug-eluting counterpart is a single sinusoidal cobalt-nickel component that winds helically from one end of the stent to the other with two or three welds between adjacent “hoops”.
Accelerated bend fatigue testing apparatus
To compare device durability, stents were subjected to accelerated cyclic bend testing carried out under contract by Medical Device Testing Services (MDT, Minnetonka, MN, USA). Fifteen examples of each 3.0 mm diameter stent design were tested. One end of a nominally deployed stent was mounted on a fixed mandrel while the other end was mounted onto a mandrel suspended in a flexible membrane (Figure 2). To minimise variability, all stents were mounted so that a connector was aligned with the 12 o’clock position on the fixed mandrel.
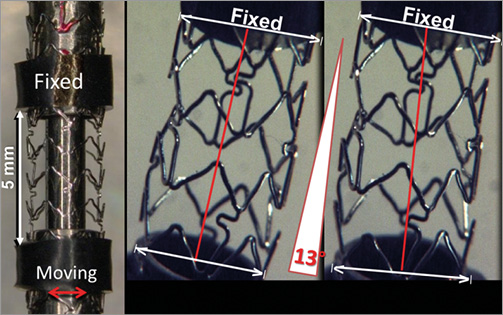
Figure 2. Accelerated bend test apparatus. One end of each stent was placed on a fixed mandrel. The other end, mounted in a mandrel suspended in a flexible membrane, was translocated ±3.5 mm in a direction perpendicular to the long axis of the stent at a frequency of six cycles/second (red double-headed arrow). The length of stent between the mandrels was 5.0 mm (red lines). Periodic microscopic inspections were made until a fracture was observed or until 10 million cycles were completed.
The mobile end of the stent was translated perpendicular to the longitudinal axis of the stent a distance of ±3.5 mm at a rate of 6 Hz to impart repeatable stent bending (Moving image 1). The distance between the fixed mandrel and moving mandrel at the flexible membrane was 30 mm so that the angle the stent bends is 13°. The frequency of 6 Hz was chosen because a high frequency saves time and cost. However, this frequency is not so rapid that the test apparatus becomes unstable. The stents were subjected to 10 million cycles in a Bose ELF 3220 (Bose Corporation, Eden Prairie, MN, USA) test instrument (MDT SGT 9120-004 and MDT SGT 9120-005 with ELF load frame) in a controlled temperature air environment. For engineering purposes the endurance limit level or “infinite” life is often defined as one million cycles17 but we tested ten times more cycles. If periodic microscopic inspections revealed a fracture, no further inspections were made of that stent. Fracture location was marked on a fracture map. This test was intended to simulate the clinical condition of cyclic bending of the transition between overlapped stents (fixed mandrel) and an adjacent single stent (moving mandrel). This method allowed for comparison of the number of cycles required to cause stent strut fracture for different stent designs. In addition, the site of strut fracture was recorded to shed light on design factors that might predispose to fracture.
Statistics
Descriptive statistics of the data are provided as mean±SD/median (IQR). Pairs of stents were compared using the Mann-Whitney U test. Statistical analyses were performed using SAS statistical software, version 9.3 (SAS Institute, Cary, NC, USA). All p-values resulted from two-sided tests and a p-value of <0.05 was considered statistically significant.
Results
The number of cycles to fracture for the Vision (288,411±193,391) and the MULTI-LINK 8 (314,475±239,869) were significantly greater than for the BioMatrix Flex design (38,904±13,160), p<0.001 (Figure 3). The difference between Vision and MULTI-LINK 8 is not significant (p=0.79). The Element, PREMIER, and Integrity designs had not fractured by 10 million cycles (Figure 3).
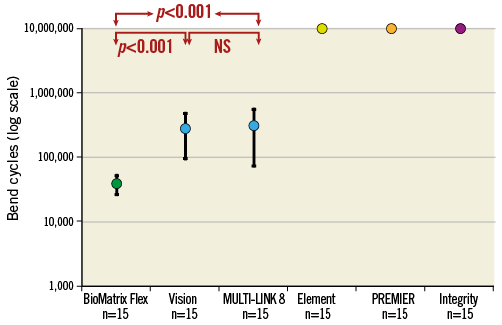
Figure 3. Mean and SD of number of bend cycles to fracture for the six stent designs. With this methodology, the BioMatrix Flex design was the most susceptible to fracture. The Vision and MULTI-LINK 8 required a factor of ten more cycles for fracture. The Omega/Element, PREMIER and Integrity designs did not fracture up to 10 million cycles. The scale of the vertical axis is logarithmic.
With the BioMatrix Flex design, all the fractures were in the “S” shaped connectors between the first (largely fixed) hoop and the second hoop (Figure 4, Figure 5). There were 27 strut fractures because 12 of the stents had two fractures so that, as there are only two connectors connecting adjacent hoops with this design, these 12 stents were completely transected.
With the Vision design (Figure 4, Figure 5), 16 of the 19 fractures occurred in the connectors between hoops two and three. Two fractures occurred in connectors between hoops three and four. There was one hoop fracture and that was in hoop two. Four of the 15 stents had two fractures but the stents were not transected because this design has three connectors between hoops.
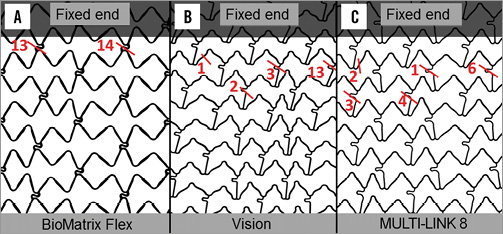
Figure 4. Visicon scans of the three designs that fractured showing sites of fracture. The fixed end of the stent is labelled. The Element, PREMIER and Integrity designs did not fracture up to 10 million cycles. The BioMatrix Flex (A), Vision (B) and MULTI-LINK 8 (C) designs all had one or more fractures per stent tested. The short red line indicates the site of fracture and the number represents the sum of fractures at that locus.
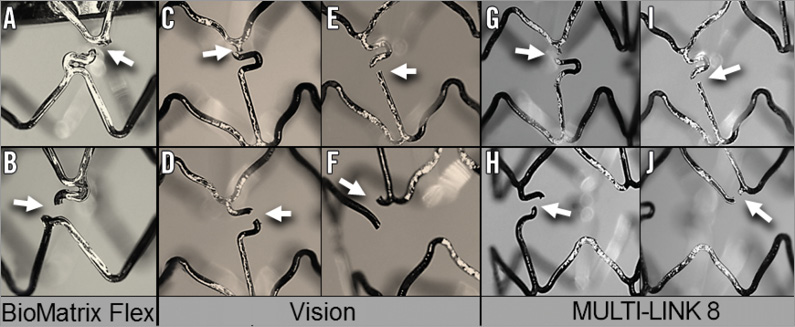
Figure 5. Representative images of the strut fractures (arrows) for the three designs which fractured. The BioMatrix Flex design fractures were in the “S” shaped connector (A & B). For the Vision and MULTI-LINK 8 designs, all but three fractures were in the connectors. They were proximal to the “U” shaped portion of the connector (C & G), in the “U” (D & H), or distal to the “U” as in E & I. Hoop fractures are shown in F & J.
Seven of the 16 fractures in the MULTI-LINK 8 were in the links (Figure 4, Figure 5) between hoops two and three. Seven fractures occurred between hoops three and four. There were two hoop fractures which were in hoop two. Only one of 15 stents of this design had two fractures.
With DES strut fracture, in addition to the damage to the metal, there is damage to the polymer (Figure 6).
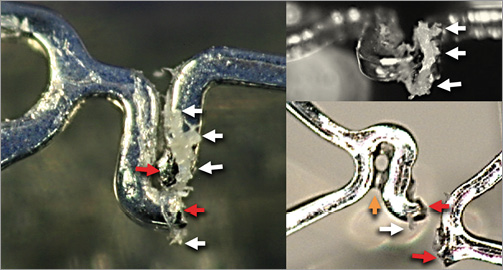
Figure 6. Strut fracture and polymer damage. The red arrows indicate the separated fracture strut ends. White arrows point to damaged polymer. The yellow arrow indicates polymer bridging between struts.
Discussion
The principal findings of this study comparing susceptibility to fracture in six different contemporary DES designs using an accelerated repetitive bend test were:
1. The stent design most susceptible to fracture was the BioMatrix Flex with struts fracturing in all stents between 10 thousand and 100 thousand cycles.
2. The number of cycles to fracture Vision and MULTI-LINK 8 stent struts was mostly between 100 thousand and one million, which was significantly more than for the BioMatrix Flex.
3. The Element, PREMIER and Integrity designs did not fracture up to 10 million cycles.
Almost all strut fractures occurred in connectors between hoops.
Strut fracture has been recognised for more than 10 years7 with at least six classification systems ranging from a single strut fracture to extreme with complete stent transection8,18-22.
While strut fracture with bare metal stents (BMS) is reported13-16, most reports are with DES. In a meta-analysis of eight studies with 5,321 patients and 108 stent fractures, the incidence of stent fracture per patient was 4% and all but one were in first-generation sirolimus-eluting CYPHER stents2. In the SIRIUS trial the strut fracture rate was 1.4% for CYPHER9. In an autopsy study of 177 consecutive lesions with first-generation DES, stent fracture was documented in 51 (29%)18. While historically most reports of fracture were of CYPHER, reports of fracture of second-generation cobalt-chromium everolimus-eluting stents (CoCr-EES) are now emerging5,6,10,23,24. In a clinical study5, CoCr-EES were implanted in 1,339 lesions in 1,035 patients. Stent fracture, defined as separation of the stent detected by fluoroscopy or intravascular ultrasound, at six to nine months, was found in 39/1,339 lesions (2.9%) and 39/1,035 (3.8%) of patients. In a post-mortem study10, the CoCr-EES was compared with first-generation SES and PES in humans. CoCr-EES had the least frequency of stent fracture (CoCr-EES=13%, SES=40%, PES=19%; p=0.007 for CoCr-EES versus SES), but fracture-related restenosis or thrombosis was comparable between the groups (CoCr-EES=6.5%, SES=5.5%, PES=1.2%).
There are challenges in the diagnosis of strut fracture. Cineangiography, the most commonly used modality, may detect fracture when there is partial or complete separation of stent ends but may not detect single strut fractures. The lesser radiopacity of some contemporary thin-strut stents compared with older thick-strut stents may impair fracture detection. StentBoost imaging (Philips Healthcare, Best, The Netherlands), through motion correction of frames from conventional angiography, can enhance detection. Computed tomography has been reported to be more sensitive for stent fracture detection than cineangiography25.
Some apparent stent fractures are not real and are called pseudo fractures, being due to longitudinal deformation where struts are pushed apart by, for instance, a post-dilating balloon11,26,27. The diagnosis of fracture using IVUS or OCT largely depends on identification of stent gaps or strut overlap28,29, both of which may be caused by longitudinal deformation11.
Strut fracture (SF) is important because it can cause undesirable clinical outcomes such as restenosis, aneurysms, stent thrombosis, acute coronary syndromes, target lesion revascularisation, and intimal hyperplasia, and may lead to death2,4-6,16,18,30,31. In a meta-analysis of eight studies2, where 108 stent fractures were recognised in 5,321 patients with DES, the probability of TLR was increased with strut fracture compared with no fracture (17% vs. 5.6%, p<0.01). In a clinical study with CoCr-EES5, the rates of myocardial infarction and target lesion revascularisation were significantly higher in the SF group than in the non-SF group (5.1% vs. 0.4%; p=0.018, and 25.6% vs. 2.0%; p<0.001, respectively). In addition, stent thrombosis was more frequently observed in the SF group than in the non-SF group (5.1% vs. 0.4%; p=0.018). Major adverse cardiac events within nine months were significantly higher in the SF than in the non-SF group (25.6% vs. 2.3%; p<0.001)5. Adverse clinical outcomes are related to the extent of strut fracture, and in a post-mortem study most adverse events were associated with the most severe grades of fracture18.
The patient and procedural risk factors for strut fracture include implantation in the right coronary artery, long stents, stent overlap, duration of implantation, ostial location, vessel angulation, bypass graft, lesion calcification, active hinge points, and complex anatomy2,5,8,9,30-32.
Stent factors that relate to fracture are the properties of the bulk metal, stent design, and antiproliferative drug elution. Endurance limit (stress below which failure never occurs) is correlated with yield strength (stress at which a material begins to deform plastically) and ultimate tensile strength (the maximum stress that a material can withstand while being pulled before failing or breaking)17,33. The newer stent alloys are less susceptible to fatigue and fracture than stainless steel. The yield strength (in megapascals [MPa]) of stainless steel (275) is less than that of nickel-cobalt (414), platinum-chromium (480) or cobalt-chromium (500). Similarly, the ultimate tensile strength (in MPa) of stainless steel (595) is less than nickel-cobalt (930), platinum-chromium (834), or cobalt-chromium (1,000)34,35.
The most important stent property in fracture resistance is stent design36. A study employing finite element analysis and a repetitive bend test to examine seven different stent designs all constructed from the same alloy, nitinol, revealed profoundly different potential for strut fracture related to differences in design36. Expanded stent flexibility relates importantly to fracture potential8. The stiff CYPHER design was particularly susceptible to fracture2,9,18,31.
The flexibility of Element and Integrity designs is greater than Vision and MULTI-LINK 8 designs35, and in the current study the Element and Integrity designs did not fracture up to 10 million cycles, whereas the stiffer Vision and MULTI-LINK 8 designs mostly fractured between 100 thousand and one million cycles (Figure 4). Strut thickness is important in fatigue resistance33 but, because of the improvements in the new alloys, stents can be constructed with thinner struts without loss of fatigue resistance. In our study, the stent constructed from stainless steel had the thickest struts (120 µm) but fractured more readily than stents constructed from newer alloys where strut thickness varied from 81 to 91 µm.
In our study, almost all fractures occurred in the curved connectors between hoops (Figure 4, Figure 5). Images from an autopsy study show that fractures of the CYPHER stents were in the connectors20. Another autopsy study showed fractures of the XIENCE (Vision design) were in connectors6. The bends in connectors are designed to increase stent flexibility but can also create areas of stress concentration which impact negatively on fatigue performance. The three designs that did not fracture, Element, Promus PREMIER and the Integrity, do not have curved connectors in contrast to those that did fracture. In general, designs with two connectors are more flexible and less susceptible to fracture than those with more. However, other factors such as the fatigue resistance of the bulk metal play a role, as the 3.0 mm BioMatrix Flex design with two connectors was the most susceptible to fracture in our study. Stent design is a balance of desirable characteristics. Alteration of one design feature such as the reduction in the number of connectors between hoops may improve flexibility and resistance to stent fracture but at the expense of stent longitudinal integrity11. Understanding the design features which limit stent fracture may assist in future stent design.
The higher incidence of clinical strut fracture in DES compared with BMS18,37 could be partly explained by selection bias due to use of DES in more complex disease known to be associated with fracture37. In addition, the localised stress imparted on non-apposed struts or areas of incomplete or absent neointimal coverage, such as may occur with DES, probably differs from that on stent struts apposed to the vessel wall and embedded in 100 to 200 µm of neointima as might be found with BMS37. An accelerated durability in vitro bending study comparing six CYPHER stents that had been covered with 200 µm silicone (to mimic intimal coverage) with six that had not been covered, showed that those covered had a reduced risk of strut fracture, suggesting that intimal coverage protects from fracture.
The mechanism of restenosis associated with strut/stent fracture in some instances may be due to distortion or acquired underexpansion38. Strut fracture is associated with intimal hyperplastic tissue in some studies, but strut fracture sites do not always develop restenosis4. In an OCT and angiographic study, strut fracture was seen after SES implantation at 11-month follow-up in 14 of 110 stents. The strut fracture group had a much higher binary restenosis rate than the non-fractured group (29% vs. 6%, p<0.02)29. In a post-mortem case report, a fractured XIENCE stent was associated with inflammation and fibrin accumulation, which the authors postulated might be related to sloughed polymer at the site of fracture6. However, in contrast, in a larger pathological study10 neointimal thickness was similar between lesions with stent fracture and those without, and there was no significant difference in fibrin deposition and inflammatory score, giant cell and eosinophil infiltration.
Percutaneous or surgical revascularisation may be necessary depending on symptoms or evidence of ischaemia. Many strut-fracture-related restenoses have been treated by repeat stenting8. While repeat stenting may produce symptomatic relief, the vessel conditions that predisposed to fracture persist and may be worsened, as overlapped stents themselves predispose to stent fracture. If strut fracture is an incidental finding without symptoms or objective evidence of ischaemia, then revascularisation is not necessary8,31.
In the future, if resorbable scaffolds become widely used, their programmed dismantling may render permanent metallic stent fracture a thing of the past.
Limitations
Bench testing may not accurately predict stent behaviour in humans. In vivo, stents are exposed to forces causing flexion, compression, and torsion, but we test only flexion. The stents were not subjected to radial loading. Length and rotational displacement of the stent were not measured. However, the flexible membrane was a highly compliant latex material that allows some movement in both the longitudinal and rotational directions. Because the angle change is only 13 degrees, the potential for change in length is relatively small, and a portion or all of this length change would be absorbed by the flexible membrane. We believe it is unlikely that stent rotation would be induced by the test apparatus but any rotation would be small in magnitude.
Another limitation is that we have tested only six different designs and 15 examples of only 3.0 mm diameter examples of each design.
We bent stents repetitively through an angle of 13° regardless of the stiffness of the design. More force is required to bend stiffer stents a prescribed distance so that stiffer designs may be disadvantaged by subjection to greater loading. In vivo, the bending force is independent of stent design and we would expect a stiff or a flexible stent to be subjected to the same loading during the cardiac cycle. However, we have had difficulty designing a test apparatus that is based on applying the same bending force to stents of different design. Nonetheless, first-generation tests have provided important insights into stent design11 which have led to modifications of stent design and subsequent evolution of testing methods that have provided further insights12.
While our bend test is a simplification of the clinical environment, all stents are subjected to similar conditions in the test apparatus. In addition, the sites of fracture seen with our study are similar to those observed clinically6,18. Furthermore, while our testing was performed by the independent testing organisation MDT, the method has similarities to testing performed by a device manufacturing company39.
Conclusion
Stent design plays a critical role in propensity for strut fracture. Almost all fractures occurred in the curved section of connectors which is where stress is concentrated. Stent designs with high expanded stent flexibility were less likely to fracture than those with less flexibility. Stent designs with fewer connectors were in general less likely to fracture than those with more because connector number is inversely related to flexibility. Stents constructed from the newer alloys of cobalt-chromium, platinum-chromium and nickel-chromium were less susceptible to fracture than the design constructed from stainless steel, in part because of the properties of their construction metal.
Understanding factors associated with late fracture may assist in improving stent design. It may aid the interventional cardiologist in selection of appropriate DES, especially in situations where there may be increased fracture risk.
| Impact on daily practice After stenting, late events may be due to restenosis, neoatherosclerosis or thrombosis, which in turn may be caused by stent strut fracture. Our repetitive bend test revealed profound differences between different stent designs in potential for strut fracture, with most fractures occurring in curved portions of connectors between hoops. Our observations may be one factor influencing stent selection and may aid manufacturers in designing stents. |
Funding
Testing was funded by the Auckland Heart Group Charitable Trust. Some stents were supplied by device companies.
Conflict of interest statement
J. Ormiston is an advisory board member for Abbott Vascular and Boston Scientific and has received minor honoraria from them. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. An Element design with one end fixed and the other moving so that the stent is bent 13º between maxima of movement at a frequency of 6 Hz.

