
Introduction
Coronary artery calcification (CAC) poses a challenge for successful percutaneous coronary intervention. CAC is associated with worse periprocedural and long-term clinical outcomes because of difficult device delivery and inadequate stent expansion. Atherectomy can effectively ablate and modify calcified plaques, optimising procedural outcomes. The Diamondback 360® Coronary Orbital Atherectomy System (Cardiovascular Systems, Inc., St. Paul, MN, USA) is indicated for lesion preparation of de novo severely calcified coronary lesions before stent implantation. Orbital atherectomy (OA) employs an eccentrically mounted 1.25 mm diamond-coated crown that utilises centrifugal force to orbit at either 80,000 or 120,000 rpm. OA has a unique bidirectional mechanism and allows continuous antegrade flow during atherectomy. Robust data on the most commonly reported complications and failure modes associated with OA are limited. We analysed the post-marketing surveillance data from the US Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE) database to report these endpoints.
Methods
The MAUDE database stores major adverse event reports involving medical devices. Reporting can be mandatory (manufacturers, importers, and device-user facilities) or voluntary (healthcare professionals, patients, and consumers). Established in the 1990s, the database is updated monthly with medical device reports containing information on the device, event date, whether the device was returned to the manufacturer, user, and manufacturer’s narrative of the event. Events are classified, on the basis of severity, into four categories: death, injury, malfunction, or other. Two independent reviewers queried the database from September 2016 to September 2018, for OA.
Results
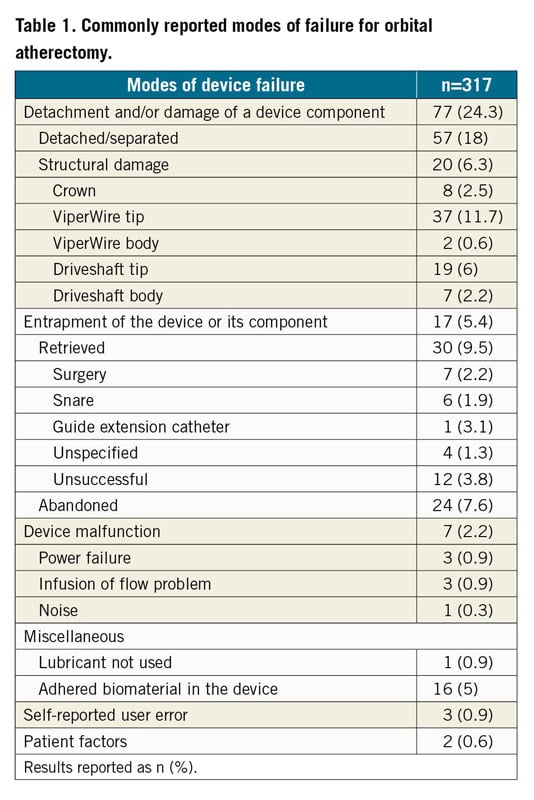
Our search yielded 520 OA device reports. After excluding reports for peripheral interventions and duplicate reports, 317 reports for coronary OA devices were included in the final analysis. Percentages represent the proportion of the total number of MAUDE reports submitted. Of OA devices with reported complications, 60 were returned to the manufacturer for analysis. The most commonly reported complications included vascular complications (perforation and dissection), arrhythmias, and death (Figure 1). The perforations were treated as follows — stent, 53.9%; surgery, 13%; covered stent, 10%; balloon angioplasty, 9.3%; and coil embolisation, 2.1% — whereas the dissections were managed as follows — stent, 58.3%; surgery, 10.4%; and balloon angioplasty, 6.25%. Pericardial drainage was required in 73.5% of pericardial effusions. Stent issues included stent thrombosis and dislodgement. The most common arrhythmias included ventricular tachyarrhythmias (47.3%) and pulseless electrical activity (31.5%). A temporary pacemaker was required in 2.8% of MAUDE reports. Death was reported in 21.8% of device-related adverse events. The most commonly reported failure modes included detachment and/or structural damage of the device component (24.3%) and device entrapment (5.4%) (Table 1). Three reports indicated user error; the operators underwent retraining on proper technique. The most commonly reported target vessel was the left anterior descending artery (Supplementary Figure 1).
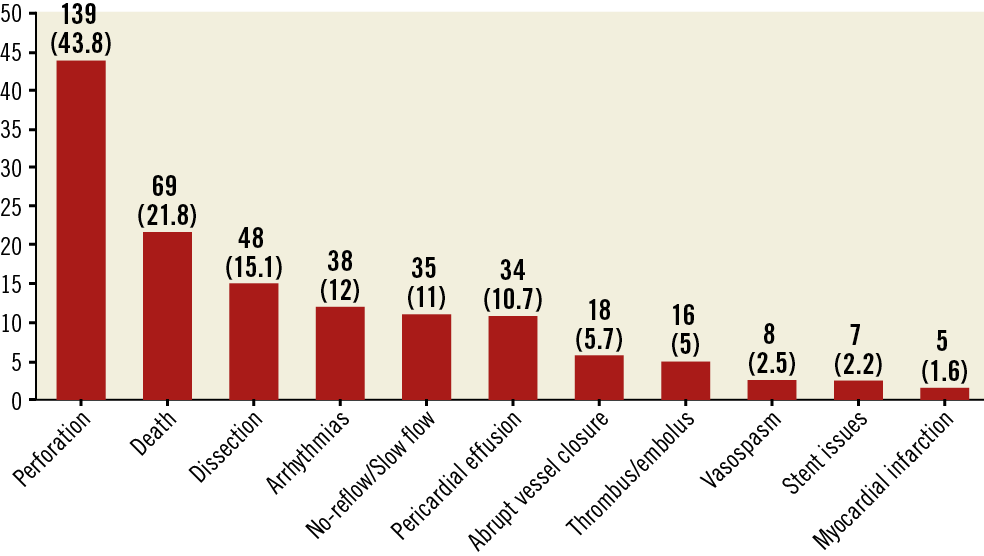
Figure 1. Summary of complications among reports submitted to the MAUDE database. Results reported as n (%). Percentages represent the proportion of reported events and do not reflect incidence rates.
Discussion
Our analysis highlights important complications associated with OA devices, for which data are limited. In clinical practice, the majority of patients with CAC are treated with conventional high-pressure balloon angioplasty and/or scoring-cutting balloon followed by drug-eluting stents. However, calcified lesions respond poorly to such therapies, resulting in limited luminal gain, suboptimal stent expansion, and adverse outcomes. Thanks to the introduction of adjunctive modalities, such as rotational and orbital atherectomy devices and intravascular lithotripsy, routine treatment of calcified lesions has become more feasible, safe, and effective. The pivotal ORBIT II trial for OA reported a one-year major adverse cardiovascular event rate of 16.9%, including cardiac death (3.2%), myocardial infarction (10.6%), perforation (1.8%), and target vessel revascularisation (5.8%) in patients with severely calcified lesions1. In a retrospective analysis of 458 patients treated with OA followed by stenting (including high-risk patients who were not surgical candidates), 30-day complications were as follows: primary endpoint of major adverse cardiac and cerebrovascular events (1.7%), all-cause mortality (1.3%), myocardial infarction (1.1%), stroke (0.2%), stent thrombosis (0.9%), dissection (0.9%), perforation (0.7%), and no-reflow (0.7%)2. A recent study comparing procedural complications related to OA and rotational atherectomy demonstrated higher dissection/perforation with OA (1.6% vs 0.3%, p=0.02)3. Our analysis provides insights into the mechanism of device-related complications but cannot verify causality. Also, this analysis does not provide information regarding the incidence rates for individual complications. With the anticipated commercial launch of OA in Europe, operators can learn from prior experience in patient selection and initial device use in the USA, thereby lowering the threshold of the learning curve and maintaining safety. For example, upon the initial approval of OA, two low-speed runs followed by an additional high-speed run were generally recommended. Collective experience has led to a change in practice, such that now the high-speed setting is often avoided in tortuous lesions, severe angulations, and vessels smaller than 3.0 mm in diameter. Some proposed techniques to avoid complications with OA include the following: maintain 1:1 motion between the crown and advancer knob, slow/steady traverse rate of 1 mm/s (rapid advances increase the risk of perforation/dissection, and the device’s unique mechanism mandates a different technique than is used with rotational atherectomy); maintain maximum single-run times to <30 seconds and total run time to <5 minutes with rest time between runs ≥prior run time; and always use low speed for the initial pass, and escalate to high speed only as needed1,2.
Limitations
Without on-site evaluation, a cause-effect relationship cannot be established between the device and adverse events. Incidence rates for each complication could not be determined because of lack of a known denominator. A minority of the devices were returned to the manufacturers for evaluation post procedure, preventing a complete analysis of failure modes. Some general limitations of the MAUDE database include underreporting (especially for complications caused by operator error), duplicate reporting, and lack of event adjudication.
Conclusion
Analysis of the MAUDE database demonstrates that, in real-world practice, OA devices are associated with important complications. With the worldwide commercialisation of OA devices, standardised complication and failure reporting policies may improve patient selection, operator proficiency, and existing device technology for optimal patient safety and outcomes.
|
Impact on daily practice Despite advances in interventional techniques, management of coronary artery calcification remains challenging. There is a need for ongoing surveillance of safety profiles, patient outcomes, and failure modes for orbital atherectomy devices. The US Food and Drug Administration Manufacturer and User Facility Device Experience database serves as an important platform for both manufacturers and clinicians to improve device performance and physician training and to optimise clinical outcomes. |
Conflict of interest statement
R. Waksman reports having served on the advisory boards of Abbott Vascular, Amgen, Boston Scientific, Cardioset, Cardiovascular Systems Inc., Medtronic, Philips Volcano, and Pi-Cardia Ltd.; serving as a consultant for Abbott Vascular, Amgen, Biosensors, Biotronik, Boston Scientific, Cardioset, Cardiovascular Systems Inc., Medtronic, Philips Volcano, Pi-Cardia Ltd.; having received grant support from Abbott Vascular, AstraZeneca, Biosensors, Biotronik, Boston Scientific, Chiesi; having served on the Speakers Bureau of AstraZeneca and Chiesi; and being an investor in MedAlliance. T. Rogers reports being a consultant and proctor for Medtronic and being a proctor for Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

