
Abstract
Aims: Percutaneous coronary intervention (PCI) of severe coronary artery calcification (CAC) is challenging. The ORBIT II study demonstrated the safety and efficacy of orbital atherectomy (OA) in patients with severe CAC. Microparticulate liberated during OA may disturb the coronary microcirculation. In the present study, we evaluated OA treatment in patients with left ventricular systolic dysfunction.
Methods and results: Patients were grouped by left ventricular ejection fraction (LVEF): 26-40% (n=33), 41-50% (n=90), and >50% (n=314). Procedural success was similar (LVEF 26-40%: 90.9%, LVEF 41-50%: 88.9%, LVEF >50%: 88.4%). Rates of major adverse cardiac events (MACE), defined as cardiac death, myocardial infarction, and target vessel revascularisation, were similar in the LVEF 26-40%, 41-50%, and >50% groups, respectively, at 30 days (9.1%, 7.8%, 11.5%) and one year (18.2%, 19.1%, 16.0%). Although the 30-day cardiac death rate was 0% in patients with left ventricular dysfunction, one-year cardiac death was higher compared with patients with preserved left ventricular systolic function.
Conclusions: No patient with left ventricular systolic dysfunction experienced cardiac death at 30 days suggesting that OA was well tolerated without haemodynamic complication. However, one-year cardiac death was higher in patients with left ventricular systolic dysfunction, consistent with previous studies demonstrating the association between reduced left ventricular function and increased mortality after PCI. ClinicalTrials.gov Identifier: NCT01092416
Abbreviations
ACC/AHA: American College of Cardiology/American Heart Association
CABG: coronary artery bypass grafting
CAC: coronary artery calcification
eGFR: estimated glomerular filtration rate
IMR: index of microcirculatory resistance
IVUS: intravascular ultrasound
LVEF: left ventricular ejection fraction
MACE: major adverse cardiac events
MI: myocardial infarction
OA: orbital atherectomy
OAS: orbital atherectomy system
PCI: percutaneous coronary intervention
RA: rotational atherectomy
TLR: target lesion revascularisation
TVR: target vessel revascularisation
Introduction
Severe coronary artery calcification (CAC) is present in 5.9% to 20% of patients undergoing percutaneous coronary intervention (PCI)1,2. Despite advances in interventional equipment and techniques, treating severe CAC remains a challenge, as it is associated with adverse outcomes including restenosis, target lesion revascularisation (TLR), vessel dissection, failure to deliver a stent or asymmetric stent expansion, balloon ruptures, undilatable lesions, death, and myocardial infarction (MI)3-11. Suboptimal stent expansion may increase the likelihood of stent thrombosis and restenosis12. Coronary artery rupture may occur due to repeated high-pressure inflations to ensure complete stent expansion13. Additionally, long-term ischaemic outcomes are significantly worse for patients with severely calcified coronary lesions compared to non-calcified lesions1,2. Patients with a low left ventricular ejection fraction (LVEF) have an increased risk of adverse events after PCI, including death14-16.
The ORBIT II study evaluated the safety and efficacy of the Diamondback 360® Coronary Orbital Atherectomy System (OAS) (Cardiovascular Systems, Inc., St. Paul, MN, USA) in preparing de novo, severely calcified coronary lesions for stent placement and is the largest clinical study to date reporting exclusively on patients with severely calcified lesions. Major findings in the ORBIT II study included successful stent delivery in 97.7% of patients, residual stenosis of <50% observed in 98.6% of patients, and low rates of angiographic complications17. Adverse event rates up to one-year follow-up, including non-Q-wave and Q-wave MI, TLR, and death, were also much lower compared with prior studies evaluating severely calcified coronary lesions17,18. Although the OAS is a safe option for difficult-to-treat patients with various comorbidities, its performance in patients with left ventricular systolic dysfunction has not been studied17. We investigated the impact of left ventricular systolic dysfunction on clinical outcomes in patients with severe CAC who underwent orbital atherectomy.
Materials and methods
STUDY DESIGN AND PATIENTS
The FDA mandated that ORBIT II be completed as a single-arm study since no comparable devices were approved for use in severely calcified coronary arteries in the USA. Details about the ORBIT II study design have been published previously17,18. Men and women, at least 18 years of age, with a de novo, severely calcified coronary lesion in a native coronary artery were enrolled in the study. Key inclusion criteria included: 1) target vessel reference diameter ≥2.5 mm and ≤4.0 mm with 70% to <100% stenosis or 50%-70% stenosis with evidence of clinical ischaemia; 2) target lesion length no longer than 40 mm; and 3) fluoroscopic or intravascular ultrasound (IVUS) evidence of severe calcium deposit at the lesion site. Severe calcium was defined as the angiographic presence of radiopacities noted without cardiac motion prior to contrast injection involving both sides of the arterial wall in at least one location and total length of calcium of at least 15 mm and extending partially into the target lesion, or the presence of ≥270° of calcium at one cross-section via IVUS. Key exclusion criteria included: 1) target vessel had a stent from previous PCI; 2) angiographically confirmed evidence of more than one lesion requiring intervention, unless the treatment of the lesions was staged; 3) target vessel was excessively tortuous; 4) patient had a recent MI (defined as CK-MB >1x upper limit of lab normal) within one month prior to procedure; 5) evidence of current LVEF ≤25% (where current was defined as the latest LVEF measurement completed within the last six months); and 6) NYHA Class III or IV heart failure. Only one target lesion was treated per subject. The operational treatment time was limited to five minutes per device, and each individual treatment interval was limited to 30±3 seconds. After the plaque modification, stent implantation was required.
The clinical study was conducted in the USA as per Good Clinical Practice and the applicable Code of Federal Regulations, and was approved by each institutional review committee. Sub-analyses were performed for patients in three LVEF subgroups (LVEF 26-40%, 41-50%, and >50%).
DEVICE DESCRIPTION
The Coronary Orbital Atherectomy System is a percutaneous device designed to reduce severely calcified plaque prior to stent deployment (Figure 1). The eccentrically mounted diamond-coated crown sands the plaque while rotating over the ViperWire Advance® Coronary Guidewire (Cardiovascular Systems, Inc.) to restore lumen patency. The crown’s orbital diameter expands radially via centrifugal force, and an increase in rotational speed allows an increase in luminal gain. The soft tissue flexes away from the crown while the hard/calcified component of the plaque is reduced with each pass of the crown (Moving image 1).

Figure 1. Diamondback 360 Coronary Orbital Atherectomy System (OAS) device. Coronary OAS device handle and 1.25 mm classic crown.
ENDPOINTS
The primary safety endpoint was major adverse cardiac events (MACE) at 30 days. MACE was defined as comprising cardiac death, MI, and target vessel revascularisation (TVR). Myocardial infarction was defined as creatine kinase-myocardial band level >3x the upper limit of normal with or without a new pathologic Q-wave. Target vessel revascularisation was defined as any repeat revascularisation of the target vessel (inclusive of the target lesion) after completion of the index procedure. Procedural success was defined as success in facilitating stent delivery with a final residual stenosis of <50% and without in-hospital MACE. Angiographic success was defined as success in facilitating stent delivery with a residual stenosis of <50% and without the occurrence of a severe angiographic complication. An independent clinical events committee adjudicated adverse events and severe angiographic complications including persistent slow flow/no reflow and abrupt closure. An angiographic core laboratory (Cleveland Clinic Foundation, Cleveland, OH, USA) performed quantitative coronary analysis on procedural angiograms and reported minimum lumen diameter, final percent residual stenosis, as well as the presence and type of dissections and perforations.
STATISTICAL ANALYSIS
Statistical analyses were performed with either the SAS software system (SAS Institute Inc., Cary, NC, USA) or R (R Core Team-2012 [R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/]).
Patient demographics, medical history, risk factors, pre- and post-procedure lesion characteristics, procedure characteristics, and outcome variables were summarised using descriptive statistics for continuous variables presented as mean±standard error and frequency tables or proportions for discrete variables. Data were compared using the Wilcoxon rank-sum test for continuous parameters and Fisher’s exact test for categorical parameters. Kaplan-Meier methods were used to obtain estimates of the 30-day and one-year event rates; p-values were calculated using Cox proportional hazards models. A multivariable Cox proportional hazards regression model was fit to assess the relationship of LVEF and one-year cardiac death after adjusting for other baseline covariates. The baseline covariates included in the model were: history of coronary artery bypass grafting (CABG), gender, history of diabetes, and history of MI.
Results
PATIENT DEMOGRAPHICS AND LESION CHARACTERISTICS
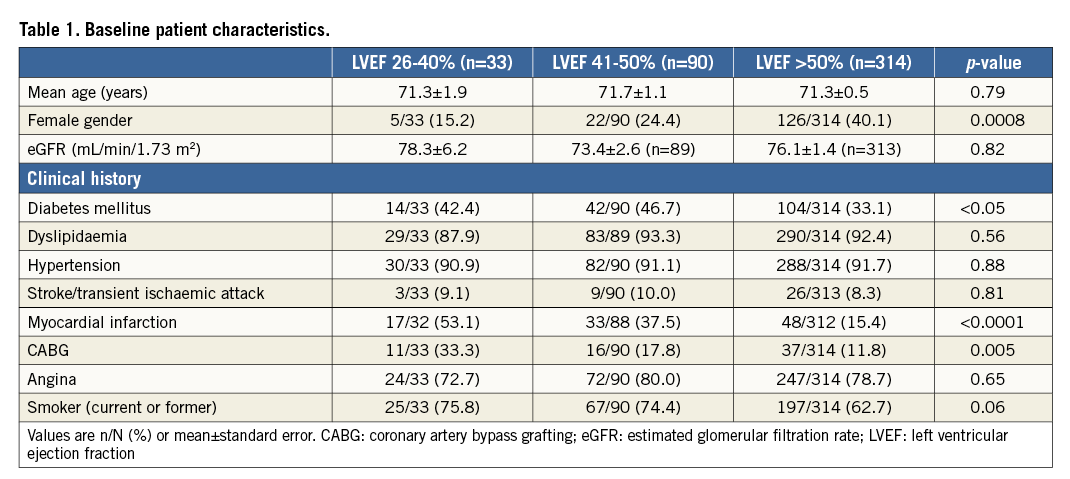
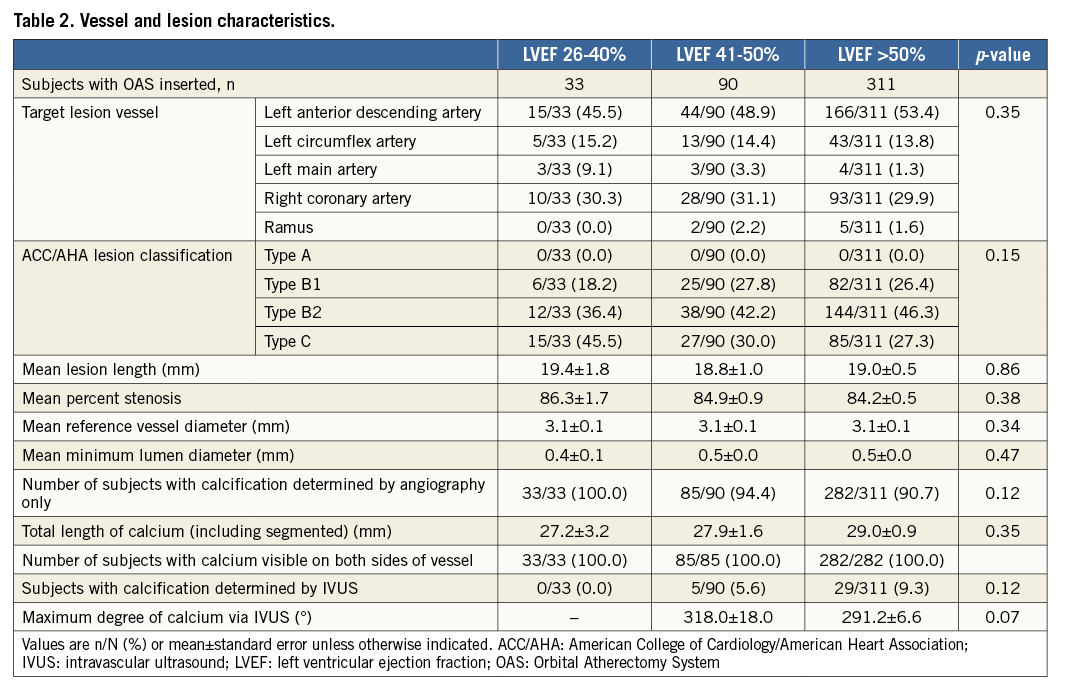
The ORBIT II study population comprised 443 patients enrolled at 49 sites. Approximately 99% (437/443) of ORBIT II patients had LVEF assessed at baseline. Patients were grouped by LVEF: 26-40% (n=33), 41-50% (n=90), and >50% (n=314) (Table 1). Patients with left ventricular systolic dysfunction were more likely to be male (p=0.0008) had a higher prevalence of diabetes mellitus (p<0.05), history of MI (p<0.0001), and history of prior CABG (p=0.005). No significant differences in vessel and lesion characteristics were noted among the LVEF subgroups (Table 2).
PROCEDURAL RESULTS
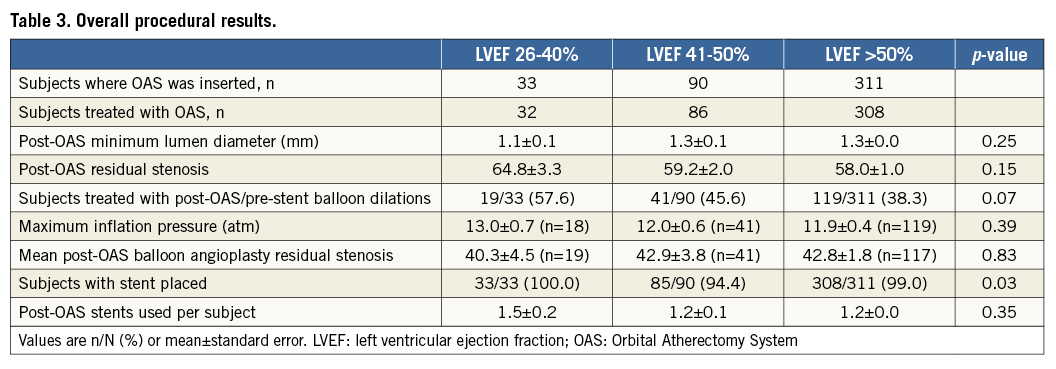
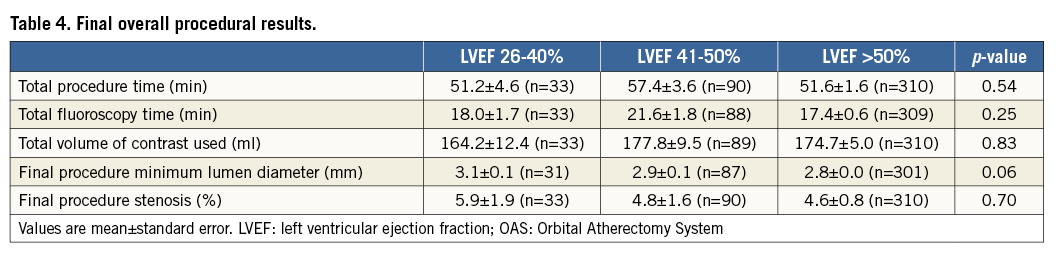
Procedural success was similar in LVEF subgroups (LVEF 26-40%: 90.9%, LVEF 41-50%: 88.9%, LVEF >50%: 88.4%; p=1.0). No significant differences in overall procedural results (Table 3, Table 4) were observed among the LVEF subgroups.
SAFETY
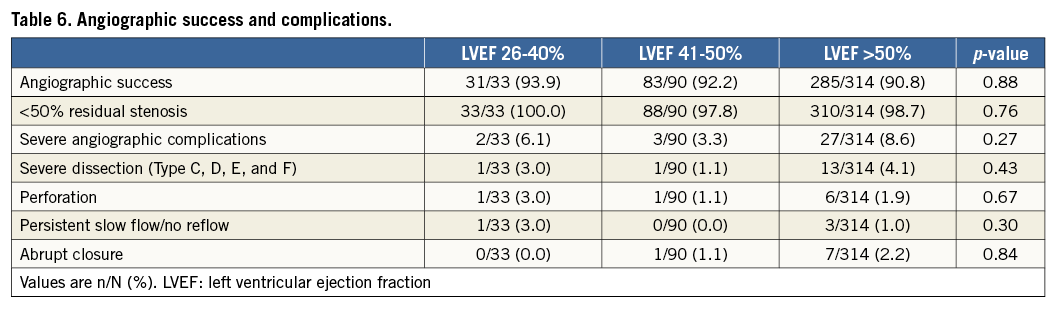
The in-hospital and 30-day MACE rates were similar in the LVEF subgroups (Table 5). The Kaplan-Meier estimates of one-year major adverse cardiac events (MACE) are shown in Figure 2. The MACE rate at one year was similar among the LVEF subgroups (p=0.83) (Figure 2A), as was MI (p=0.55) (Figure 2C), and target vessel revascularisation (p=0.70) (Figure 2D). However, cardiac death was increased in patients with left ventricular systolic dysfunction, with a stepwise increase in mortality with lower LVEF (LVEF >50%: 1.6%, LVEF 41-50%: 6.9%, LVEF 26-40%: 9.1%, p=0.02) (Figure 2B). The results of the multivariable model indicate that, even after adjustment for baseline covariates, there was a statistically significant difference across LVEF subgroups in the risk of cardiac death at one year (p=0.01). Angiographic success and severe angiographic complications were not significantly different among the LVEF subgroups (Table 6).
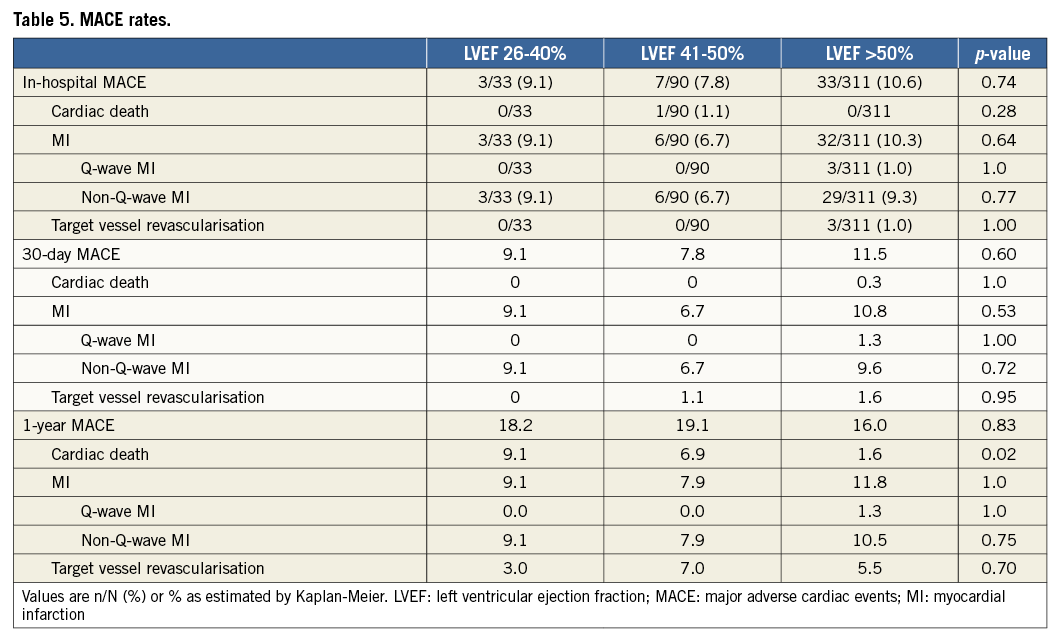
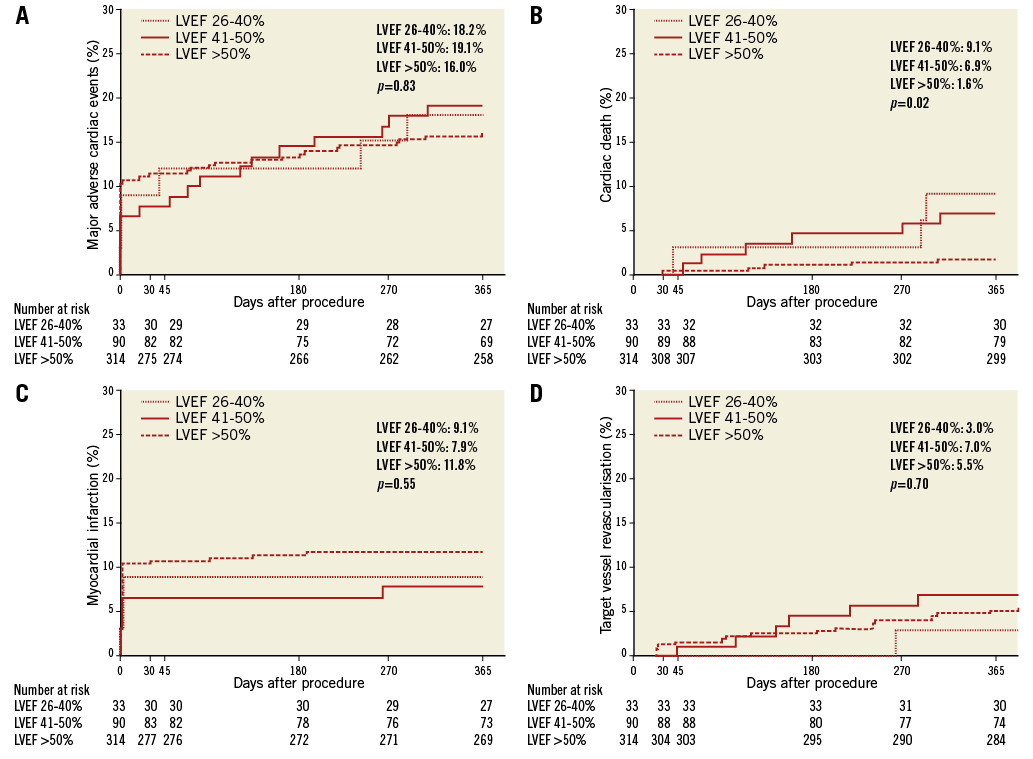
Figure 2. Kaplan-Meier estimates of one-year major adverse cardiac events (MACE). Estimates of major adverse cardiac events (MACE) (A), including cardiac death (B), myocardial infarction (C), and target vessel revascularisation (D) are presented for patients with left ventricular ejection fraction (LVEF) volume 26-40%, LVEF 41-50%, or LVEF >50%.
Discussion
Current treatment options for severely calcified coronary lesions include PCI and CABG. Compared to non-calcified lesions, percutaneous or surgical revascularisation of severely calcified lesions is associated with an increased risk of death and adverse events1,2,19,20. Recently published data demonstrate that orbital atherectomy treatment of severely calcified coronary lesions prior to stent placement is a promising strategy for these historically difficult-to-treat lesions17,18,21. In light of the recent findings of the STICH trial related to the survival benefit associated with CABG in coronary artery disease patients with heart failure and severe left ventricular systolic dysfunction, we sought to understand the outcomes of OAS-mediated PCI not only in patients with severely calcified lesions but also in those with left ventricular systolic dysfunction22,23.
The main finding of this sub-analysis of the ORBIT II study was that patients with severe CAC and left ventricular systolic dysfunction who underwent orbital atherectomy had similar MACE rates at one-year follow-up as well as procedural results and angiographic success compared with patients with preserved left ventricular systolic function. Although there were no cardiac deaths in patients with left ventricular systolic dysfunction at 30 days, cardiac death at one year was higher compared with patients with preserved left ventricular systolic function.
The in-hospital and 30-day MACE rates, which were predominantly driven by periprocedural non-Q-wave MI, were similar in the three groups. No patients with left ventricular systolic dysfunction had a Q-wave MI within 30 days of the procedure. Furthermore, no patients with left ventricular systolic dysfunction suffered Q-wave MI at one-year follow-up. At one year, the rate of MI was similar in all three groups.
In-hospital and 30-day TVR did not occur in any patient with left ventricular systolic dysfunction. The rate of TVR at one year was similar in the groups. Only one patient (3.1%) with LVEF 26-40% had TVR at one year.
No patients with left ventricular dysfunction experienced cardiac death periprocedurally or within 30 days post procedure. Patients with left ventricular systolic dysfunction were able to tolerate orbital atherectomy without significant haemodynamic complications, despite reduced physiological reserve and microparticulate debris liberated during orbital atherectomy which may disturb the coronary microcirculation. However, patients with left ventricular systolic dysfunction had increased cardiac mortality compared with patients with preserved left ventricular systolic function at one-year follow-up. An analysis of >230,000 PCI procedures reported a strong relationship between left ventricular function and mortality following PCI, with worsening left ventricular function associated with worse 30-day and longer-term mortality24. Analysis from the National Heart, Lung, and Blood Institute-sponsored Percutaneous Transluminal Coronary Angioplasty and Dynamic Registries, National Cardiovascular Data Registry, and the HORIZONS-AMI trial also demonstrated that impaired left ventricular function predicted mortality25-27.
Assessment of left ventricular function is helpful to risk stratify patients undergoing PCI. Inclusion of left ventricular function as one of six clinical parameters to the anatomical SYNTAX score (to form the SYNTAX II score) enhanced the ability of the model to predict four-year mortality following percutaneous or surgical revascularisation28. Each 10% increase in left ventricular function was independently associated with a 44% reduction in four-year mortality after PCI.
The effect of left ventricular systolic dysfunction has not been adequately studied with other atherectomy devices for severe CAC. The data with rotational atherectomy in patients with left ventricular dysfunction are limited. In a retrospective study of 23 patients with LVEF <30% who underwent rotational atherectomy, MACE-free survival at 30 days was 100%29. Long-term clinical outcomes were not reported. The mechanism of action of orbital atherectomy is different from rotational atherectomy (RA); the OAS utilises an orbital motion instead of a drill motion like that of RA. The orbital motion allows continuous flushing of particulate instead of one bolus release, as seen with RA. This difference in mechanism of action probably results in the lower rates of slow flow/no reflow with OAS (0.7%-0.9%) as compared to Rotablator™ (0%-29.9%) (Boston Scientific, Marlborough, MA, USA)17,21,30-34.
Study limitations
The ORBIT II study was designed as a non-randomised, prospective clinical study and lacks a control arm. Future studies are needed to examine the efficacy of the OAS versus other PCI treatments. The ORBIT II study was not powered to determine OAS efficacy and safety in this sub-analysis. In addition, the number of patients with left ventricular systolic dysfunction was low. Patients with severe left ventricular dysfunction (LVEF ≤25%) were excluded from the ORBIT II study and therefore the outcomes of these patients are unknown. Data on the concomitant use of haemodynamic support devices such as intra-aortic balloon pump or Impella® (Abiomed, Danvers, MA, USA) were not available.
Conclusions
In the first and only assessment of patients with left ventricular dysfunction who underwent orbital atherectomy for severe CAC, the MACE rate at one-year follow-up was similar compared with patients with preserved left ventricular systolic function. There were no cardiac deaths in patients with left ventricular systolic dysfunction at 30 days, suggesting that patients tolerated orbital atherectomy without any significant haemodynamic complications. However, cardiac death at one year was higher in patients with left ventricular systolic dysfunction, consistent with previous studies reporting reduced left ventricular function and increased mortality after PCI. Left ventricular function should be assessed prior to orbital atherectomy given its importance in risk stratification to quantify risk accurately.
| Impact on daily practice In the ORBIT II study of orbital atherectomy treatment for severely calcified coronary lesions prior to stent placement, patients with left ventricular systolic dysfunction who underwent orbital atherectomy had a similar MACE rate at one-year follow-up as well as procedural results and angiographic success compared with patients with preserved left ventricular systolic function. Left ventricular function should be assessed prior to orbital atherectomy given its importance in risk stratification to quantify risk accurately. These findings suggest that orbital atherectomy may be an appropriate revascularisation strategy in patients with left ventricular systolic dysfunction and severe coronary artery calcification. |
Acknowledgements
The authors thank the rest of the ORBIT II investigators. The authors thank Nissa J. Mollema, PhD, and Ann Behrens, BS, of Cardiovascular Systems, Inc., for editing and critical review of this manuscript.
Funding
Cardiovascular Systems, Inc., sponsored the ORBIT II study and financially supported the statistical analysis for the left ventricular ejection fraction sub-analysis.
Conflict of interest statement
M. Lee, A. Lee, R. Shlofmitz, E. Shlofmitz, and J. Chambers have consulting agreements with Cardiovascular Systems, Inc. B. Martinsen is employed by and owns stock in Cardiovascular Systems, Inc.
Supplementary data
Moving image 1. Animation of FDA-approved Coronary Orbital Atherectomy System.
Supplementary data
To read the full content of this article, please download the PDF.
Animation of FDA-approved Coronary Orbital Atherectomy System.

