
Abstract
Aims: Recently, favourable procedural 30-day and one-year outcomes with the Diamondback 360 Orbital Atherectomy System (OAS) in the treatment of severely calcified lesions have been reported. The purpose of this study was to assess the therapeutic mechanism and efficacy of the OAS with optical coherence tomography (OCT) imaging.
Methods and results: This was an observational imaging study in 18 patients with complex calcified coronary artery lesions who underwent percutaneous coronary intervention with the OAS. Pre-OAS and post-OAS OCT analyses demonstrated that the minimum lumen area (MLA) increased from 2.07±0.66 mm2 to 2.38±0.68 mm2 with a lumen volume increase of 9.68±17.22 mm3 in the ablated segment with a length of 30.7±13.1 mm. The maximal vessel injury (dissection) involved the intima in 39% and the media in 6% of the study population. To eliminate the influence of post-OAS vasoconstriction, the ablation area was measured with the interpolated original lumen surface by comparing the endoluminal border of the pre-OAS image. The ablation area at the maximal ablated cross-section was 0.55±0.41 mm2, and the ablation volume by OAS was 2.68±2.80 mm3.
Conclusions: OAS effectively ablated coronary calcified tissue with some degree of intimal dissection. OCT imaging can be used to assess the total ablation volume after orbital atherectomy.
Introduction
Despite advances in technology, percutaneous coronary intervention (PCI) of severely calcified coronary lesions remains challenging1,2. Current therapeutic options to manage calcified lesions (e.g., cutting/scoring balloons and rotational atherectomy) are associated with a high rate of restenosis and stent failure at long-term follow-up3. Recently, the ORBIT II (Evaluate the Safety and Efficacy of OAS in Treating Severely Calcified Coronary Lesions) trial demonstrated favourable procedural, 30-day and one-year outcomes with the Diamondback 360® Coronary Orbital Atherectomy System (OAS) (Cardiovascular Systems, Inc., St. Paul, MN, USA) in the treatment of severely calcified lesions, resulting in its Food and Drug Administration (FDA) approval4,5. OAS uses the principle of off-axis centrifugal force, with the orbital motion diameter being proportional to the applied speed. This orbital movement results in sanding of the entire calcified wall and might allow greater blood flow (non-occlusive) with less heat generation and thermal injury during the procedure compared with a potentially more aggressive and occlusive atherectomy approach6. However, adjunctive intracoronary imaging, such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT), was not evaluated in the previous trials4,5. Additional data from these imaging techniques would be highly informative and would provide insights into the therapeutic mechanisms and effects of OAS7,8. OCT especially is a high-resolution technology allowing a detailed assessment of the coronary vessel wall9. In this study, we evaluated the effect of OAS with OCT to elucidate the therapeutic mechanism and efficacy of OAS.
Methods
STUDY DESIGN AND POPULATION
This was an observational imaging study in patients with documented myocardial ischaemia and complex calcified native coronary artery lesions. The study was approved by the institutional review board at St. Francis Hospital, The Heart Center, Roslyn, New York. Between March 2014 and July 2015, 18 severely calcified coronary artery lesions in 18 patients were treated by OAS with pre- and post-OAS OCT evaluations which were then analysed in this study. Inclusion criteria were: i) age above 18 years, ii) angiographically proven coronary artery disease, including the following criteria: target reference vessel diameter between 2.5 and 4.0 mm by visual estimation, luminal diameter reduction of 70% to 99% by visual estimation, severe calcification of the target lesion, iii) angina II to IV using the Canadian Cardiovascular Society classification criteria and/or reproducible ischaemia in the target area by ECG or scintigraphy. Exclusion criteria were: i) unprotected left main lesions, ii) severe tortuosity of the lesion, iii) target lesion thrombus.
STUDY DEVICE
The coronary OAS manufactured by Cardiovascular Systems, Inc. (CSI) is used to facilitate stent delivery in patients with coronary artery disease who are acceptable candidates for percutaneous coronary angioplasty or stenting due to de novo, severely calcified coronary artery lesions. The system includes a single-use, 6 Fr catheter with an eccentrically mounted 30 μm solid diamond-coated crown on a coil-wound flexible drive shaft covered by a protective sheath. The crown shaft tracks and rotates over a guidewire, the ViperWire Advance® (CSI), which can be independently advanced. The coronary OAS utilises a diamond-coated, 1.25 mm crown that offers the following advantages: 1) differential sanding, allowing healthy tissue to flex away; 2) coronary plaque reduction by changing the lesion compliance, enabling successful stent deployment; 3) production of minuscule particulates that do not require embolic protection; 4) continuous perfusion of saline and blood during orbit. A detailed description of the novel use of centrifugal force and differential sanding employed by the coronary OAS to modify calcified coronary lesions was previously reported10.
OPTICAL COHERENCE TOMOGRAPHY ANALYSIS
The image acquisition was performed with the C7XR™ imaging console and the Dragonfly™ intravascular imaging catheter (both St. Jude Medical, St. Paul, MN, USA). After positioning the OCT catheter distally to the target lesion for OAS ablation, the catheter is pulled back automatically at 20 mm/s with simultaneous contrast infusion by a power injector (flush rate 3-4 mL/s). Images were stored and analysed offline. Analysis of the OCT images was performed with the QCU-CMS software (Medis, Leiden, The Netherlands), using the following methodology.
Pre- and post-OAS OCT analyses were performed in 0.2 mm longitudinal intervals for the entire target lesion. Pre- and post-OAS OCT images were completely matched by the position and shapes of side branches and calcified plaque. The ablated segment was defined as the segment between the proximal and distal anatomical landmarks, such as side branch or deep calcifications, which also included the entire ablated lesion by OAS observed in the post-OAS image. Lumen contour was defined as the continuous interface between blood and non-blood structure. Lumen area was defined as the area within this contour. For the quantitative evaluation of ablation volume, lumen volume change of the segment between pre-OAS and post-OAS was used as a first methodological approach. However, in this analysis, we could not eliminate the influence of vasomotion (we have observed vasoconstriction after OAS in 28% of the cases). Therefore, a second methodological approach was implemented to eliminate such potential confounding factors: the ablation area was measured with the interpolated original lumen surface by comparing the endoluminal border of the pre-OAS image (Figure 1). The ablation volume (mm3) was calculated using the disc summation method (0.2 mm slice thickness): the sum of the ablation area multiplied by 0.2 mm.
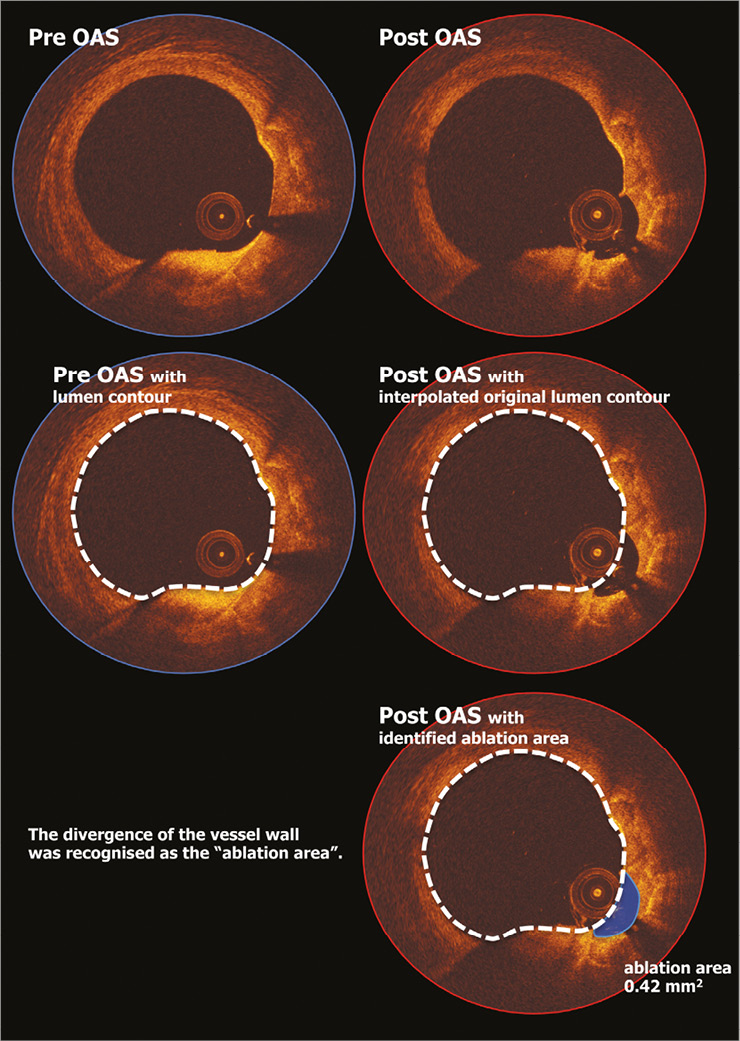
Figure 1. The OCT methodology for ablation area measurement. To eliminate the influence of post-OAS vasoconstriction, the ablation area was measured with the interpolated original lumen surface by comparing the endoluminal border of the pre-OAS image. OAS: orbital atherectomy system; OCT: optical coherence tomography
For post-stenting analysis, cross-sections at 1 mm intervals within the stented segment were analysed. Mean and minimum lumen/stent areas were measured for each stent. Proximal and distal reference lumen area was estimated as the mean lumen area between the 5 mm proximal and distal segments to the edges of the stent. The reference lumen area was defined as the average of the proximal and distal reference area. The stent expansion index was a ratio of minimum stent area to the reference lumen area. Apposition of struts was assessed by measuring the distance between the endoluminal reflective border of a strut and the lumen contour. If this distance was longer than the strut thickness (89 μm for XIENCE® [Abbott Vascular, Santa Clara, CA, USA], 91 μm for Resolute Integrity® [Medtronic, Santa Rosa, CA, USA], and 81 μm for MULTI-LINK MINI VISION® [Abbott Vascular]), it was considered as an incomplete stent apposition (ISA) strut. Struts crossing over the ostium of side branches and overlapping stents were excluded from the analysis of apposition. The percentage of ISA struts in each stented lesion was calculated as (the number of ISA struts)/(total number of struts in all cross-sections of the lesion)*100. The ISA area was defined as the space between the lumen contour and stent contour at the location of ISA struts. Mean and the largest ISA areas were evaluated at lesion level.
Location and circumferential distribution of calcium and lipid-rich plaque were quantified in OCT. Calcium was defined as a signal-poor or heterogeneous region with a sharply delineated border. The largest continuous arc of calcium and total arc of calcium in the ablated segment of the pre-OAS image were measured in degrees with a protractor centred on the lumen. Lipid-rich plaque was defined as a signal-poor region within an atherosclerotic plaque, with poorly delineated borders, a fast OCT signal drop-off, and little or no OCT signal backscattering. When the continuous arc of lipid-containing plaque was ≥90° within a plaque, it was considered to be a lipid-rich plaque11. For each lipid plaque, the lipid arc was measured at 1 mm intervals through the entire length of the ablated segment. Mean and maximal total arc of lipid-rich plaque and maximal longitudinal length in a lesion were assessed. These parameters were also evaluated in the pre-OAS matched cross-section with the maximal ablated cross-section in post-OAS images.
Dissections were classified as flaps, cavities, double-lumen dissections or fissures, and their longitudinal extensions in the ablated segment were measured9. Their severity was characterised by quantifying the dissection depth and area. Dissection depth was measured based on the previously described method12. Dissection area was measured as flap area for flaps, cavity area for cavities, and cap area for double-lumen dissections, respectively9. For fissures, due to a poor demarcation of the root of the flap-like structure, no measurements were performed on the fissures at the cross-sectional level; however, they were included in the assessment of longitudinal extension. The axial injury of the dissection was described as intimal involvement when only the intima was affected and media was still intact, as medial involvement when the dissection extended into the media without disruption of the entire medial layer, and as adventitial involvement when the media was dissected throughout its thickness. Whenever the media was not discernible, the dissection was classified as involving only the intima9.
CLINICAL OUTCOME
Procedural and in-hospital clinical outcomes were reported in this study. We defined device success as successful delivery and deployment of the stent at the intended target lesion and successful withdrawal of the delivery system with attainment of final in-stent residual stenosis of less than 50% by quantitative coronary angiography. We defined procedural success as device success without the occurrence of cardiac death, target vessel myocardial infarction, or repeat target lesion revascularisation during the hospital stay. Definitions of periprocedural myocardial infarction and stent thrombosis were based on the third universal definition13 and the Academic Research Consortium criteria14, respectively.
STATISTICAL ANALYSIS
The normality distribution for continuous data was examined with the Shapiro-Wilk test. Continuous variables with normal distribution are expressed as means±standard deviations and those with unequal variance are expressed as medians and interquartile ranges (25th and 75th percentiles). Categorical variables are expressed as numbers and frequencies. Group means for continuous variables with normal and non-normal distributions were compared using the Student’s t-test and Mann-Whitney U test, respectively. Categorical variables were compared using the χ2 test or Fisher’s exact test, where appropriate. Linear regression analysis was performed to evaluate the relationship between ablation volume by OAS and pre-OAS parameters on OCT. The intra- and inter-observer reproducibility for the ablation area at cross-section level was assessed by intraclass correlation coefficients (ICC) (95% confidence intervals [95% CI]). Statistical significance was assumed at a probability p-value of <0.05. All statistical analyses were performed with SPSS, Version 22.0.0 (IBM Corp., Armonk, NY, USA).
Results
PATIENT DEMOGRAPHICS
Baseline characteristics of the included patients are summarised in Table 1. Procedural characteristics and clinical outcomes are shown in Table 2. No complications related to OAS were observed. The procedure success rate was 100%, and no periprocedural myocardial infarction was observed. Median length of hospital stay was one day (0-5), and no stent thrombosis was observed during this period.
OCT ANALYSIS
Representative OCT images showing ablation area by OAS are presented in Figure 2. Table 3 summarises the results of OCT analysis. Post OAS, minimum lumen area increased from 2.07±0.66 mm2 to 2.38±0.68 mm2, with a lumen volume increase of 9.68±17.22 mm3. In 28% of the cases, lumen volume decreased post OAS due to vasoconstriction. Ablation area at the maximal ablated cross-section was 0.55±0.41 mm2, and the ablation volume by OAS was 2.68±2.80 mm3 (Figure 3). The intra- and inter-observer reproducibility for the measurement of ablation area at cross-section level was 0.998 (95% CI: 0.998-0.999) and 0.970 (95% CI: 0.964-0.976), respectively.
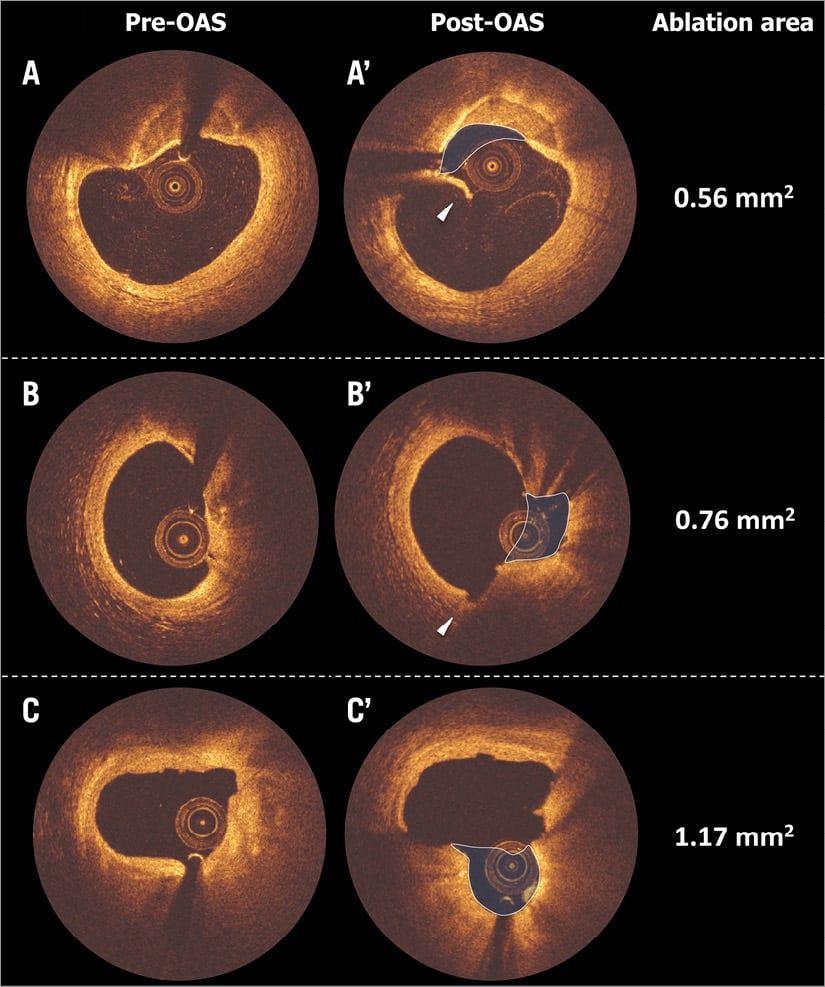
Figure 2. Representative OCT images pre-OAS and post-OAS. Three representative matched OCT cross-sections showing ablated calcified plaque (blue shadow) by OAS are presented in panels A - C (pre-OAS: A - C and post-OAS: A’ - C’). Flap after OAS is presented in panel A’ (white arrowhead). Medial dissection by OAS (observed only in this case) is presented in panel B’ (white arrowhead). Relatively mixed calcified plaque was also intensively ablated, panel C’. OAS: orbital atherectomy system; OCT: optical coherence tomography
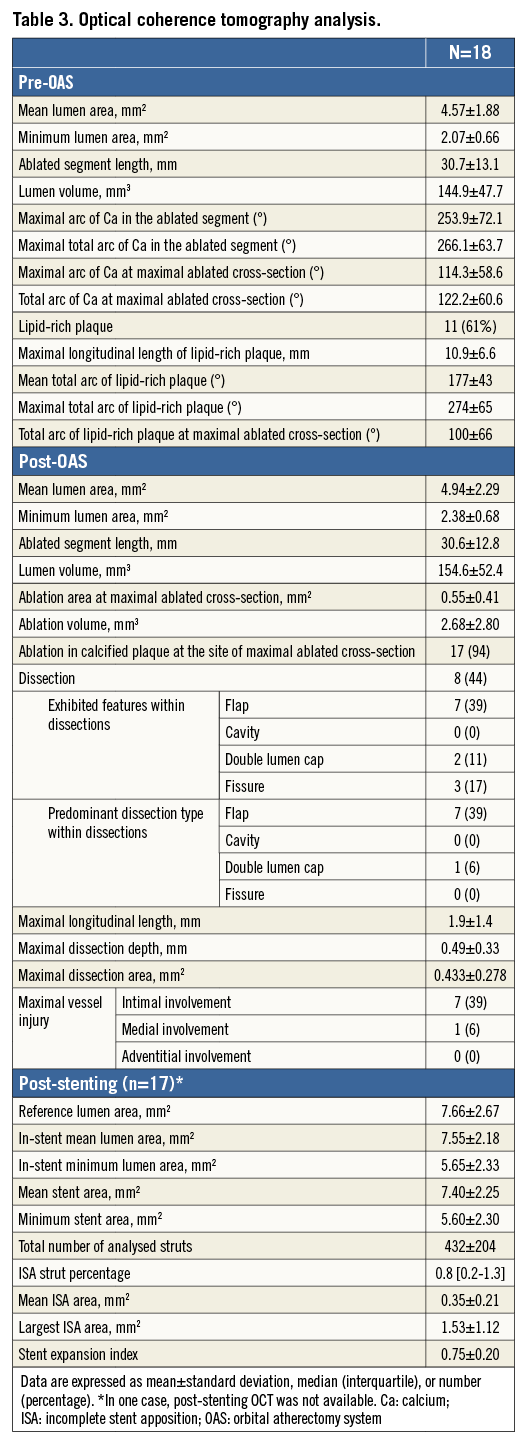
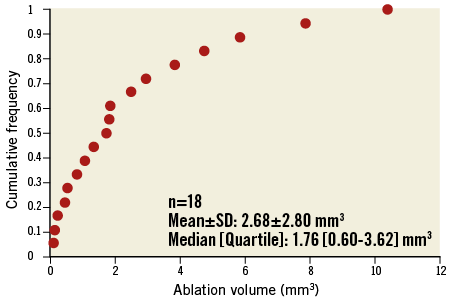
Figure 3. Cumulative frequency distribution curve of ablation volume by OAS. Ablation volume was 2.68±2.80 mm3 (mean±standard deviation), 1.76 [0.60-3.62] mm3 (median [interquartile]), ranging from 0.11 mm3 to 10.39 mm3.
At the site of the maximal ablated cross-section, the atherectomy was directed to the calcified plaque in 94% of the cases. However, there was no clear correlation between extent of calcified lesion at the maximal ablated cross-section and ablation volume (Figure 4). Smaller pre-OAS minimum lumen area contributed to larger ablation volume (R2=0.383, p=0.006).
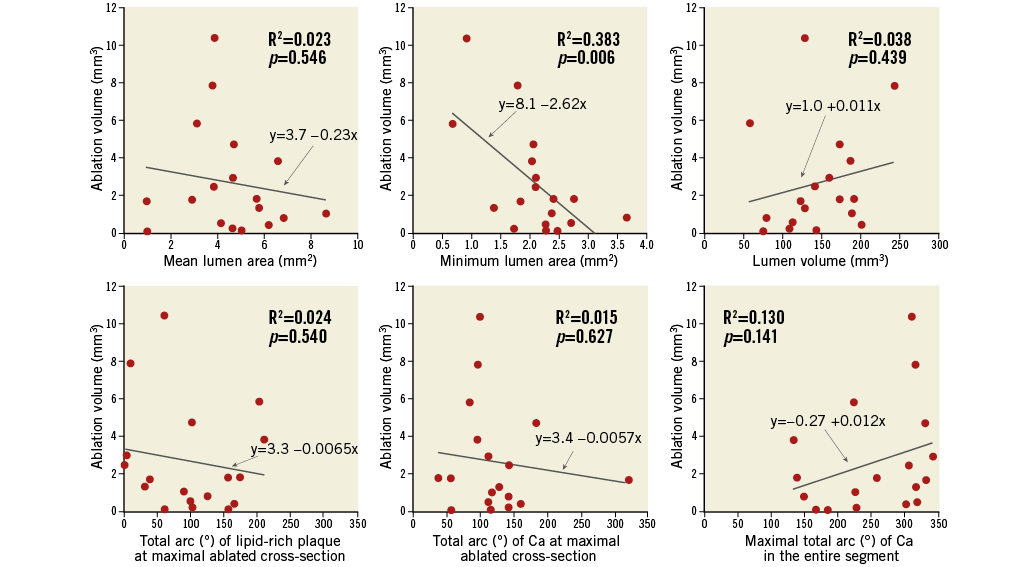
Figure 4. Relationship between pre-OAS parameters and ablation volume by OAS. Smaller pre-OAS minimum lumen area contributed to larger ablation volume (p=0.006). Other parameters were not associated with efficacy of OAS (p>0.05). Ca: calcium; OAS: orbital atherectomy system
Intimal erosion on the surface of the calcified lesion was observed in all cases. Dissection was observed in 44% of the study population. The most frequently exhibited feature within dissection was flap, which was observed in 39% of the patients. However, the maximal longitudinal length was limited to 1.9±1.4 mm, and the maximal dissection area was 0.433±0.278 mm2. The maximal vessel injury involved the intima in 39% and the media in 6% of the study population. No adventitia-involved vessel injury was observed.
Discussion
The main findings of this study can be summarised as follows: 1) the ablation volume by OAS was 2.68±2.80 mm3; 2) dissection was observed in nearly half of the cases, but vessel injury was limited to intimal involvement; and 3) OAS selectively ablated the calcium interface of the stenosis in the maximal ablated cross-section.
New strategies to deal with severely calcified plaques are always welcomed with enthusiasm by interventional cardiologists. However, as with any other new technology, the mechanisms underlying its process need to be well understood. For the ablation of calcified tissue, OAS uses a principle of off-axis centrifugal force, with the orbital motion diameter being proportional to the applied speed. This orbital movement results in sanding of the entire calcified wall and might allow greater blood flow (non-occlusive) with less heat generation and thermal injury during the procedure compared with a potentially more aggressive and occlusive atherectomy approach6.
EFFICACY AND SAFETY OF OAS
In the present study, we showed that OAS effectively and safely ablated calcified tissue. We were able to estimate the volume of the ablated tissue and to quantify the acute gain in lumen volume related to it. Since, to the best of our knowledge, this is the first time this approach has been used, the relative impact of this ablation volume is difficult to assess. However, removal of a relatively small amount of superficial calcium results in a significant increase in MLA in the treated lesions. From a vessel compliance perspective, removal of superficial calcium seems to be the most critical objective of the atherectomy treatment. It seemed to be successfully achieved by the orbital principle with one size of burr in the OAS device since the post-stenting minimum stent area in the present study was comparable even with non-calcified lesions in a recent trial15. Post-stenting minimum stent area could be the surrogate for the evaluation of the efficacy of plaque modification. Although the absolute value of MLA cannot be directly compared due to the difference in reference vessel size, the current results seem to be more or less comparable with previous reports on rotational atherectomy and cutting balloon11,16. However, as is well known, an early acute lumen gain with rotational atherectomy does not have a favourable impact on clinical outcomes, or even negatively influence late loss after stenting1. The quality of plaque modification might be one of the important key factors to achieve favourable clinical outcomes.
With regard to the safety of this technology, no severe complication was observed and the procedure success rate was 100%. The differential sanding theory, less heat generation due to non-occlusive orbital movement, and small particle size (2 μm) seemed to contribute to the favourable efficacy and safety results17.
QUALITY OF PLAQUE MODIFICATION BY OAS
We also showed the effect of OAS in the lumen surface, demonstrating that it was mainly associated with some degree of intimal dissection. The dissection rate was much lower than that described by Radu et al, who concluded that OCT-detected edge dissections, which are angiographically silent in the majority of cases, constitute a relatively frequent and benign finding, and are not associated with acute stent thrombosis or restenosis up to one-year follow-up9. A previous study with post-OAS OCT evaluation showed that it caused more extensive and deeper dissections when compared to the Rotablator™ system (Boston Scientific, Marlborough, MA, USA)11. However, in the report from Kini et al, no pre-procedural images were acquired so that no quantification of the volume of the ablated tissue could be performed. To compare the efficacy of both atherectomy systems effectively and precisely, in addition to the assessment of dissection, the estimation of the total ablated volume by comparing pre- and post-procedure OCT images, as we did in the current study, should be undertaken. The present study could not provide us with a clear answer as to the favourable clinical outcomes after OAS due to its small number of study subjects and non-comparative study design. However, the precise assessment of the quality of plaque modification performed in the present study might help to explain the favourable results in the ORBIT II trial5. Further imaging clinical studies of head-to-head comparison between OAS and other atherectomy devices would be helpful to assess the real value of OAS.
Limitations
The current study has some limitations. First, the sample size was limited and the study population was highly selected, which could impair its external validity. Second, while the OCT analytic method used for the evaluation of ablation area was quite accurate in almost all the cross-sections, in some cross-sections it was difficult to draw the interpolated original lumen contour accurately due to wire artefact (shadows in the vessel wall) and attenuation of intimal erosion. In addition, although the length assessment by OCT is substantially accurate18, the volume assessment could be influenced by measurement deviation of length since the volume was calculated using the disc summation method. Lastly, the present study had no comparison with other plaque modification techniques such as cutting/scoring balloons and rotational atherectomy. Therefore, the results should be interpreted with caution and considered hypothesis-generating.
Conclusions
OAS effectively ablated coronary calcified tissue with some degree of intimal dissection. OCT imaging can be used to estimate the total ablation volume after orbital atherectomy.
| Impact on daily practice Favourable procedural 30-day and one-year outcomes with the Diamondback 360 Orbital Atherectomy System (OAS) in the treatment of severely calcified lesions have been reported. The present study demonstrated that: 1) the ablation volume by OAS was 2.68±2.80 mm3; 2) dissection was observed in nearly half of the cases, but vessel injury was limited to intimal involvement; 3) OAS selectively ablated the calcium interface of the stenosis in the maximal ablated cross-section. These results might help to explain the mechanisms for favourable procedural outcomes seen in previous trials. |
Conflict of interest statement
Y. Sotomi is a consultant for Goodman and has received a grant from the Fukuda Memorial Foundation for Medical Research and SUNRISE lab. R. Shlofmitz has received speaking honoraria from Cardiovascular Systems Inc. and St. Jude Medical. Y. Onuma and P. Serruys are members of the Advisory Board for Abbott Vascular. The other authors have no conflicts of interest to declare.

