Abstract
Background: Recent trials support the efficacy of renal sympathetic denervation (RDN) to reduce blood pressure (BP). Nevertheless, about one third of patients are considered non-responders to RDN. Previous retrospective analyses suggest arterial stiffness could predict BP response to RDN.
Aims: We prospectively assessed the potential of invasive pulse wave velocity (iPWV) to predict BP response to RDN. Additionally, we aimed to establish non-invasive models based on arterial stiffness to predict BP response to RDN.
Methods: iPWV, magnetic resonance imaging-based markers of arterial stiffness and the carotid-femoral pulse wave velocity were recorded prior to RDN in patients with treatment resistant hypertension. Changes in daytime BP after 3 months were analysed according to the prespecified iPWV cut-off (14.4 m/s). Regression analyses were used to establish models for non-invasive prediction of BP response. Results were compared to iPWV as reference and were then validated in an external patient cohort.
Results: Eighty patients underwent stiffness assessment before RDN. After 3 months, systolic 24h and daytime BP were reduced by 13.6±9.8 mmHg and 14.7±10.6 mmHg in patients with low iPWV, versus 6.2±13.3 mmHg and 6.3±12.8 mmHg in those with high iPWV (p<0.001 for both). Upon regression analysis, logarithmic ascending aortic distensibility and systolic baseline BP independently predicted BP change at follow-up. Both were confirmed in the validation cohort.
Conclusions: iPWV is an independent predictor for BP response after RDN. In addition, BP change prediction following RDN using non-invasive measures is feasible. This could facilitate patient selection for RDN treatment.
Introduction
Effective treatment of hypertension, the major preventable cause of mortality worldwide, remains a very significant global public health concern1. Depending on the definition used, 5-30% of patients in whom blood pressure (BP) control, by means of lifestyle modification and medical treatment, fails, suffer from resistant hypertension1. In the past decade, an interventional treatment approach of catheter-based renal sympathetic denervation (RDN) has emerged as a potential alternative for these patients. Recent randomised trial results support the efficacy of this treatment in reducing BP in comparison to sham treatment2345. Nevertheless, all these trials uniformly report that about one third of patients do not experience relevant BP reduction following the intervention. To enhance the efficacy of RDN, reducing the variability of BP response to the intervention has been suggested to be of utmost importance6. Therefore, early identification of patients who benefit from RDN (responders) and those who are unlikely to respond (non-responders) seems crucial.
It has been suggested that an assessment of arterial stiffness could be useful in predicting response to RDN7. In previous studies from our group, elevated invasive pulse wave velocity (iPWV) as a surrogate for vascular stiffness has been identified as a predictor of a poor BP response following RDN8910. As estimation of iPWV requires catheterisation, non-invasive measures to estimate the success of RDN are desirable. As such, magnetic resonance imaging (MRI)-derived markers of arterial stiffening, such as ascending aortic distensibility (mAAD) and total arterial compliance (mTAC), seem to have some predictive value, but further research is necessary11. Similarly, the carotid-femoral pulse wave velocity (cPWV), as assessed using bedside tools1213, may be an easy and cost-effective method to estimate arterial stiffness, but its value in prediction of BP response to RDN is not well described.
To improve patient selection for RDN, we conducted a trial to prospectively confirm iPWV as a predictor of RDN response and to investigate the potential of different non-invasive estimates for arterial stiffness to predict a BP response to RDN.
Methods
Trial design
We conducted a prospective single-centre clinical trial in patients undergoing intravascular ultrasound RDN to:
1) confirm the value of a predefined invasive pulse wave velocity (iPWV) as a predictor of BP response after RDN (primary outcome),
2) develop prediction models for (a) linear and (b) binary (≥5 mmHg) 24h BP response after RDN, using non-invasive markers of arterial stiffness and clinical characteristics and to compare them with iPWV (exploratory outcome),
3) validate these models and findings using an external cohort of patients (validation outcome). Validation was not predefined but was implemented retrospectively.
The study was approved by the local ethics committee and was performed in accordance with the Declaration of Helsinki. All participants provided written informed consent. The trial is registered at ClinicalTrials.gov: NCT02772939.
Study participants
Patients were screened if diagnosed with resistant hypertension (office BP >160 mmHg systolic or >90 mmHg diastolic, despite treatment with 3 or more different classes of antihypertensive drugs on at least 50% of maximum dosage for hypertension, including at least one diuretic, unless intolerant to diuretics). Antihypertensive medication had to be stable for at least 4 weeks. Patients then underwent ambulatory blood pressure measurement (ABPM) to assess baseline ABPM values. Inclusion criteria were resistant hypertension with systolic daytime BP >135 mmHg on ABPM. Exclusion criteria were <18 or >75 years old, pregnancy, life expectancy <6 months, evidence of untreated secondary hypertension, known renal artery stenosis or anatomy unsuitable for interventional RDN and any main renal artery diameter <4.0 mm. Further details on study participants, BP measurement and exclusion of secondary hypertension can be found in Supplementary Table 1.
Invasive measurement of arterial stiffness
iPWV was determined by invasive measurements prior to RDN and sedation. A 7 Fr sheath was placed in the right femoral artery and a 4 Fr pigtail catheter (Cordis) in the ascending aorta. Simultaneous pressure curves were recorded through the pigtail and the sheath’s side-port. The foot-to-foot method was used to determine iPWV, as previously described10.
Magnetic resonance imaging-based assessment of stiffness
All patients underwent MRI before RDN to assess mTAC, mAAD and MRI-based pulse wave velocity (mPWV). Details of the MRI protocol as well as estimation of the different markers of vascular stiffness can be found in the Supplementary Appendix 1.
Carotid-femoral pulse wave velocity
Non-invasive values for cPWV were measured with a dedicated piezoelectric device and software (Complior Analyse; Alam Medical)13. Measurements were taken in a supine position after at least 5 minutes of rest in a quiet environment and before RDN. Two full measurements, consisting of 5-10 heart cycles, were averaged.
RDN procedure
Endovascular ultrasound RDN was performed using the Paradise catheter (ReCor Medical), a balloon-cooled device that creates a fully circumferential thermal ablation pattern using acoustic energy. Details of the procedure have been published previously2414. All procedures were performed by experienced interventional cardiologists with experience in ultrasound RDN.
Definitions
Binary blood pressure response (BBPR) was defined as reduction of ≥5 mmHg in 24h systolic BP on ABPM at 3 months, which was deemed as clinically relevant in a previous consensus statement on RDN7.
An optimal sensitivity threshold (OST) was defined as the value with a 90% sensitivity to detect BBPR after 3 months in the study cohort.
Follow-up and outcomes
At 3 months, ABPM was repeated to assess the primary endpoint. Medication changes and adherence were assessed at 1 and 3 months through structured interviews with patients.
The trial was designed to address 2 outcomes: the primary endpoint was the change in systolic daytime BP on ABPM at 3 months between patients above and below the predefined pulse wave velocity cut-off of 14.4 m/s, derived from prior retrospective analyses10. Key secondary outcomes were the areas under the receiver operator characteristics (ROC) curves and linear regression coefficients for non-invasive models to predict BP response in comparison to iPWV. We: 1) identified independent predictors and multivariable models for linear 24h BP change, 2) calculated ROC curves and OSTs for each model, 3) compared ROC curves and linear regression coefficients, 4) validated the OSTs in an external validation cohort (Central illustration).
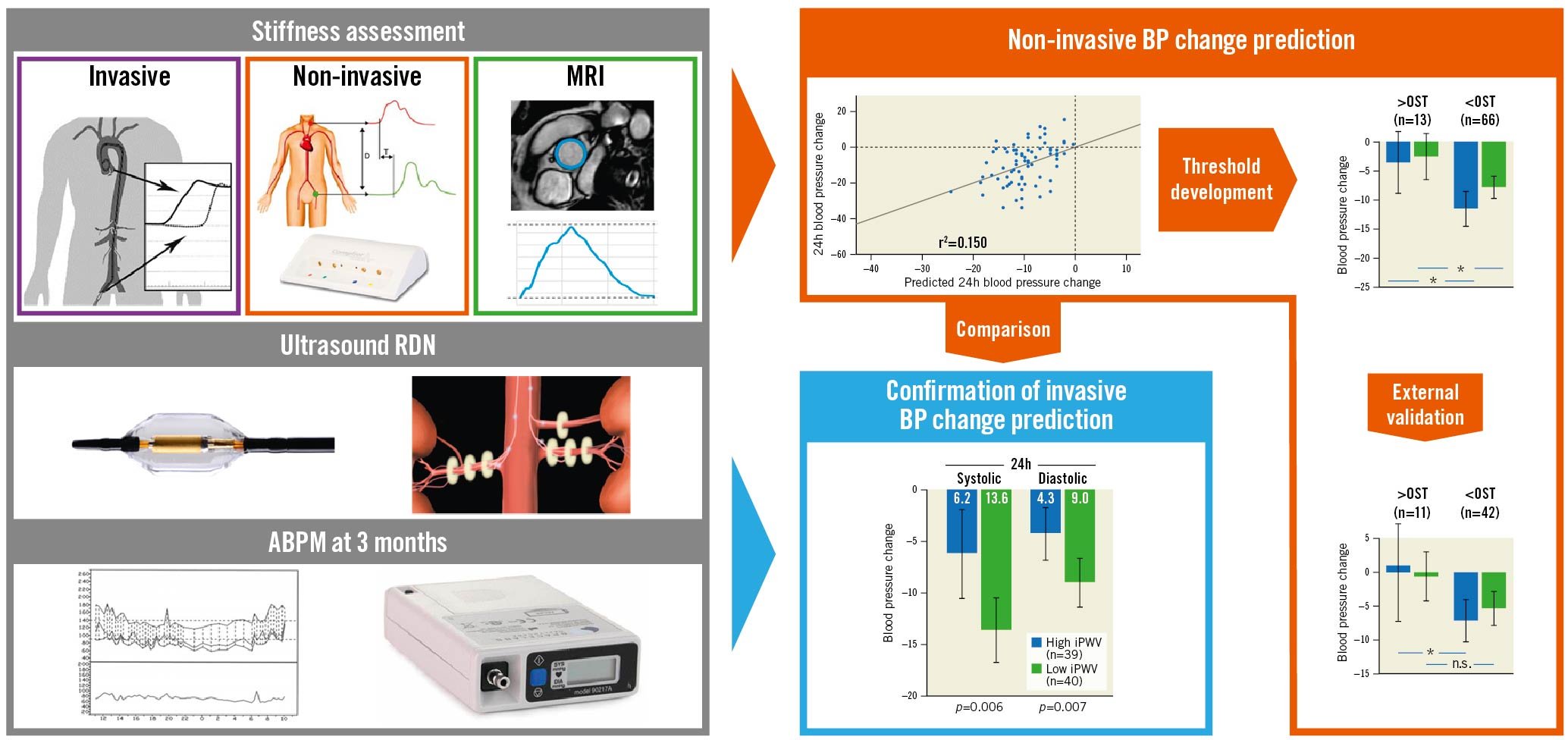
Central illustration. Prediction of RDN response in treatment resistant hypertension. *p<0.05; n.s.: not significant; ABPM: ambulatory blood pressure monitoring; BP: blood pressure; iPWV: invasive pulse wave velocity; MRI: magnetic resonance imaging; OST: optimal sensitivity threshold; RDN: renal denervation
Statistical analysis
Linear and logistic regression, ROC curves and areas under the curves (AUC) were calculated for the prediction of linear BP response and BBPR to RDN. Details on the uni- and multivariable regression analyses as well as on sample size calculation can be found in the Supplementary Appendix 1.
G-Power 3.1.9.2 was used for power calculation (University of Düsseldorf, Germany)15 . All other statistical analyses were calculated with SPSS 24.0.0.0 and 28.0.0.0 (IBM) and MedCalc 16.4.3 (MedCalc Software).
Results
Between June 2015 and May 2020, 80 patients were enrolled (Figure 1). All patients underwent bilateral RDN. One patient was lost to follow-up. Survival status for this patient was confirmed by contacting the treating general practitioner and the subject was excluded from further analysis. In total, 79 patients were available for final analyses.
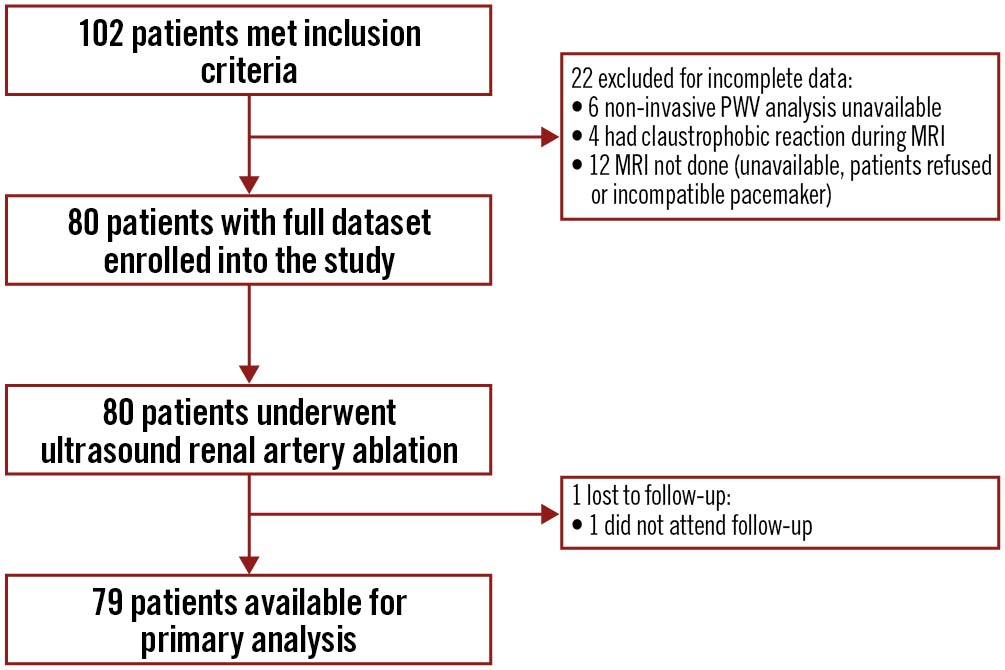
Figure 1. Study flow. MRI: magnetic resonance imaging; PWV: pulse wave velocity
Baseline characteristics and BP outcome
Clinical baseline characteristics, blood pressure and medication are shown in Table 1. Details of medication adherence can be found in Supplementary Appendix 2. At 3 months, 24h systolic and diastolic BP values were reduced by 10.0/6.7±12.2/8.0. Corresponding daytime BP values were reduced by 10.5/7.1±12.4/8.0 mmHg (p<0.001 for all). Forty-seven patients (59%) were classified as having a BBPR.
CONFIRMATION OF iPWV AS A PREDICTOR FOR BP REDUCTION
In comparing patients with low iPWV (<14.4 m/s) and patients with high baseline iPWV, baseline characteristics were balanced between the groups, except for age, which was higher in the high iPWV group (Supplementary Table 1). Patients with low iPWV had a significantly greater reduction of 24h and daytime systolic and diastolic BP (p<0.01 for all) (Figure 2).
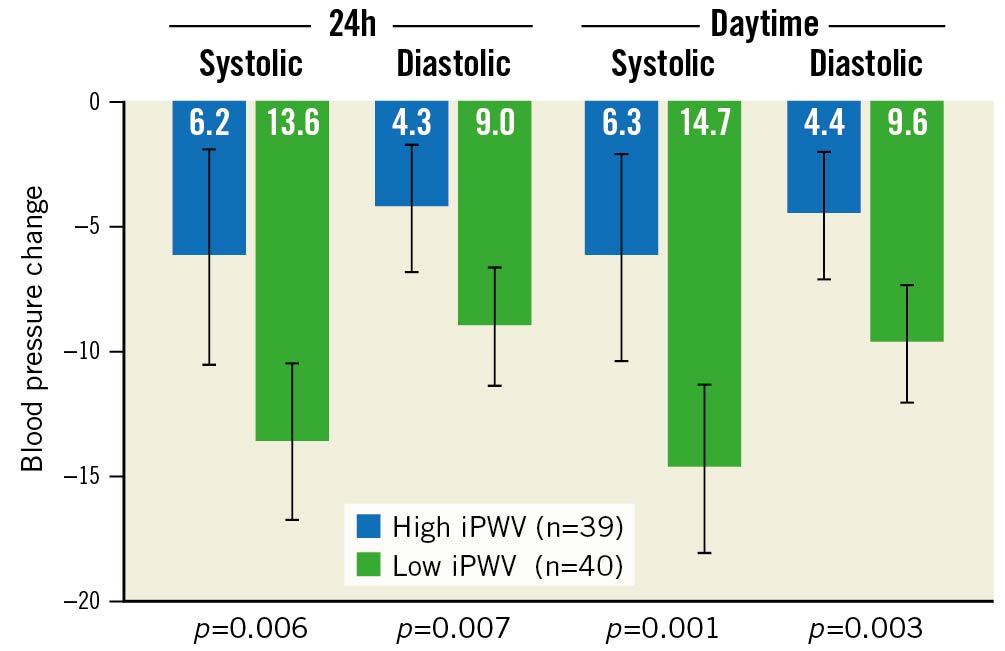
Figure 2. Primary outcome: blood pressure changes in patients with low (<14.4 m/s) vs high (≥14.4 m/s) invasive pulse wave velocity. Error bars indicate 95% confidence intervals. iPWV: invasive pulse wave velocity
In a linear regression model, iPWV was confirmed as an independent predictor for daytime and 24h BP reduction at follow-up (Beta=0.242 and 0.232) (Supplementary Table 2).
iPWV was poorly correlated with 24h systolic BP change after 3 months in a linear or logarithmic fashion (r²=0.055 and 0.076, p=0.04 and 0.01, respectively). AUC for prediction of BBPR was 0.695. BBPR frequencies were 44% in patients with high iPWV and 75% in patients with low iPWV (p=0.001). Upon logistic regression, iPWV was an independent predictor of BBPR (p=0.019).
Exploratory non-invasive blood pressure outcome prediction
With cPWV, mAAD, mPWV and mTAC, four different markers of arterial stiffness were available to establish a prediction model. Of these, only mAAD and logarithmic mAAD were univariable predictors of 24h BP reduction. Individually, both were also independent predictors of 24h BP reduction at follow-up (Table 2). Baseline 24h systolic BP, prescription of 5 or more drug classes as well as female gender were also identified as independent predictors for 24h systolic BP reduction. Serum creatinine and isolated systolic hypertension (ISH) were significantly associated with 24h systolic BP reduction upon univariable analysis but did not reach significance level in multivariable analysis (Table 2).
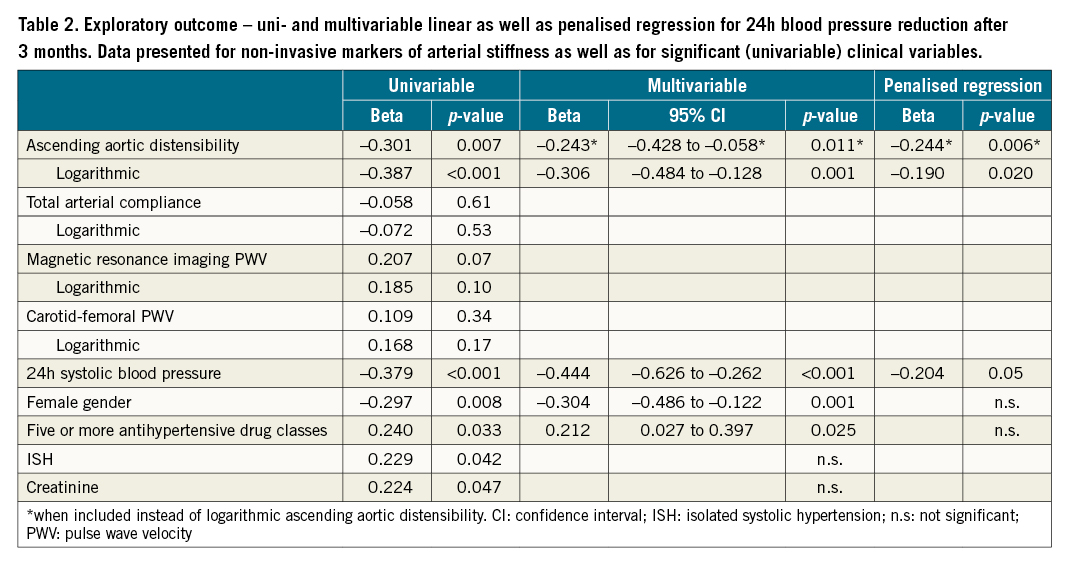
Logarithmic mAAD had a poor but significant correlation with 24h systolic BP (r²=0.150, p<0.001) and had an acceptable accuracy in predicting 24h BBPR after 3 months (AUC 0.714). Using an OST (-5.2) to detect 24h BBPR as described above, a significantly stronger systolic and diastolic BP reduction was found in patients with mAAD below, in comparison to patients above, the OST (p<0.05 for both, systolic and diastolic BP). Resulting 24h BBPR frequencies in the 2 groups were 65% and 31% (Figure 3).
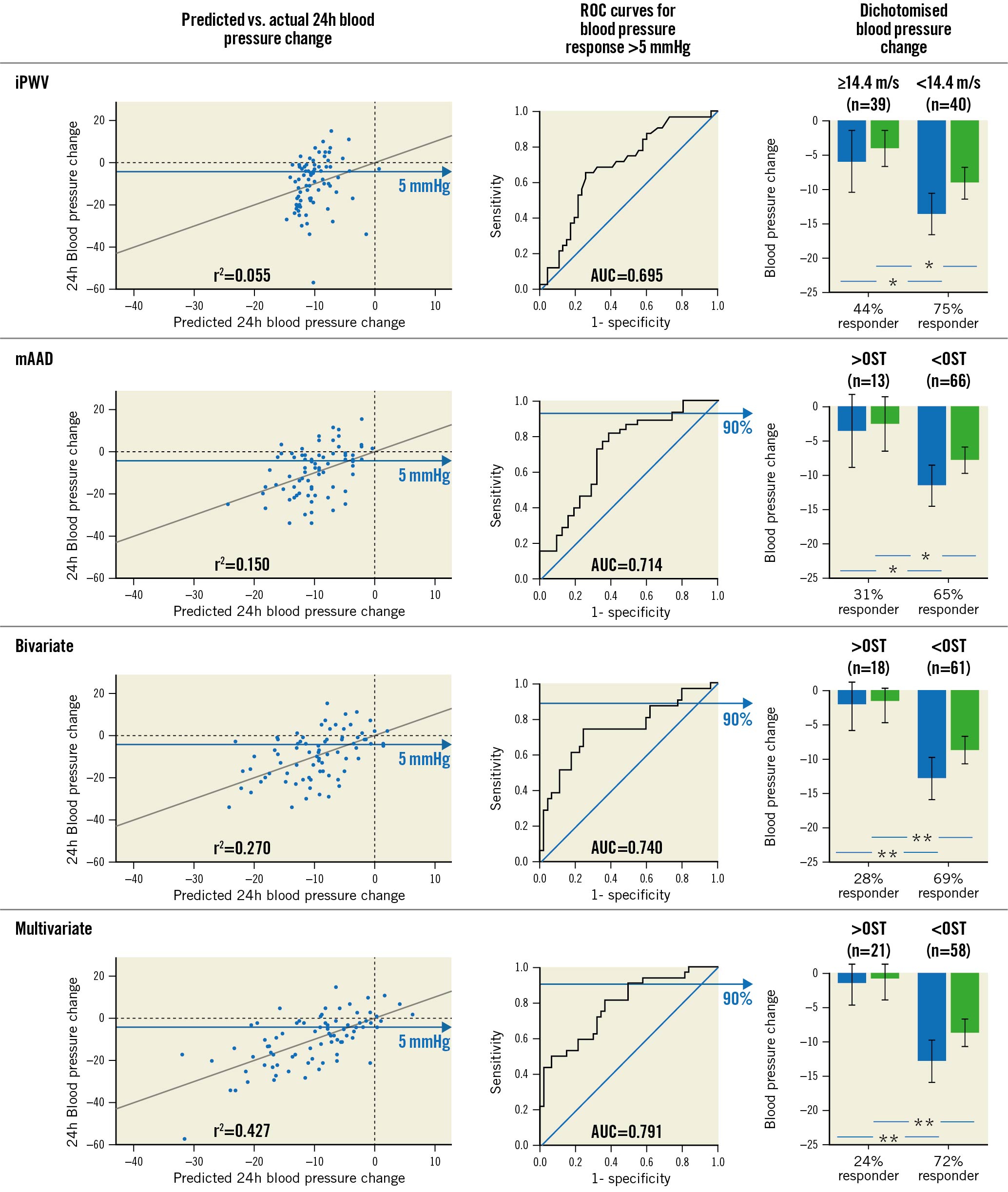
Figure 3. Exploratory outcome. Accuracy of predicted blood pressure change 3 months after RDN (left column) and prediction of a binary blood pressure response >5 mmHg (mid column) for invasive pulse wave velocity, ascending aortic distensibility, a multi- and a bivariate model. Systolic (blue) and diastolic (green) blood pressure change and 24 hr binary blood pressure responder frequencies using the predefined cut-off for iPWV and optimal sensitivity thresholds for ascending aortic distensibility, the bi- and the multivariate models (right column). *p<0.05; **p<0.001. Error bars indicate 95% confidence intervals. AUC: area under the curve; iPWV: invasive pulse wave velocity; mAAD: ascending aortic distensibility; OST: optimal sensitivity threshold; RDN: renal denervation; ROC: receiver operator characteristics
A multivariable model including logarithmic mAAD and all independent predictors as stated above, showed a modest correlation with systolic 24h BP change after 3 months (r²=0.427, p<0.001) and had an acceptable accuracy in predicting BBPR (AUC 0.791). Using an OST (-5.0), a significantly stronger systolic and diastolic BP reduction was observed in patients below versus above OST (p<0.001 for both). BBPR frequencies in the 2 groups were 72% and 24% (Figure 3). Upon logistic regression, baseline systolic 24h BP, logarithmic mAAD and intake of 5 or more drug classes were independent predictors of BBPR, while female gender was not (Supplementary Table 3). Upon penalised regression analysis for systolic 24h BP reduction, only baseline systolic 24h BP and logarithmic mAAD contributed significantly to the model (Beta=-0.204 and -0.244, p=0.05 and 0.006, respectively, regularised r²=0.334) (Table 2).
A bivariable model including 24h systolic baseline BP and logarithmic mAAD was significantly correlated with systolic 24h BP change (r²=0.270, p<0.001) and yielded an AUC for BBPR prediction of 0.740. Using an OST (-5.0), BP reduction was more pronounced in patients below versus above OST (p<0.001 for both), and corresponding BBPR rates were 69 and 38% (Figure 3). Upon logistic regression, logarithmic mAAD was an independent predictor of BBPR (p=0.002).
The cut-off values for OST as well as regression equations for each model are presented in Supplementary Table 4. Correlations between the individual measures of arterial stiffness are presented in Supplementary Table 5.
COMPARISON WITH iPWV
AUCs for BBPR prediction did not differ significantly between logarithmic mAAD, the bivariable and the multivariable model in comparison to iPWV (AUC 0.714, 0.740 and 0.791 vs 0.695, p=n.s. for all). The bivarible and the multivariable model were significantly better correlated with 24h systolic BP change after 3 months than iPWV alone (r²=0.427 and 0.270 vs 0.055, p<0.001 and p=0.04 respectively), while logarithmic mAAD was not (r²=0.150, p=0.31).
Validation
Fifty-two patients were available for validation. Mean 24h systolic and diastolic BP were reduced by -5.4±11.0 and -4.4±7.7 mmHg (p<0.001). 24h BBPR was observed in 33 patients (63%). In the validation cohort, logarithmic mAAD and 24h systolic baseline BP were confirmed as independent predictors upon linear regression analysis, while female gender and prescription of 5 or more antihypertensive drug classes were not (Supplementary Table 6). Correlation coefficients did not differ significantly between the 2 study cohorts for logarithmic mAAD and the bivariable model (r²=0.118 vs 0.150 and 0.304 vs 0.270, p=0.80 and 0.76) but were significantly lower for the multivariable model in the validation cohort (r²=0.149 vs 0.427, p=0.04).
Application of the OST values for predicted non-response from the exploratory analysis showed a significant reduction of 24h systolic BP in patients below versus above OST for logarithmic mAAD and the bivariable model (p=0.03 and 0.04) (Figure 4) while BP was not different between the groups when using the multivariable model.
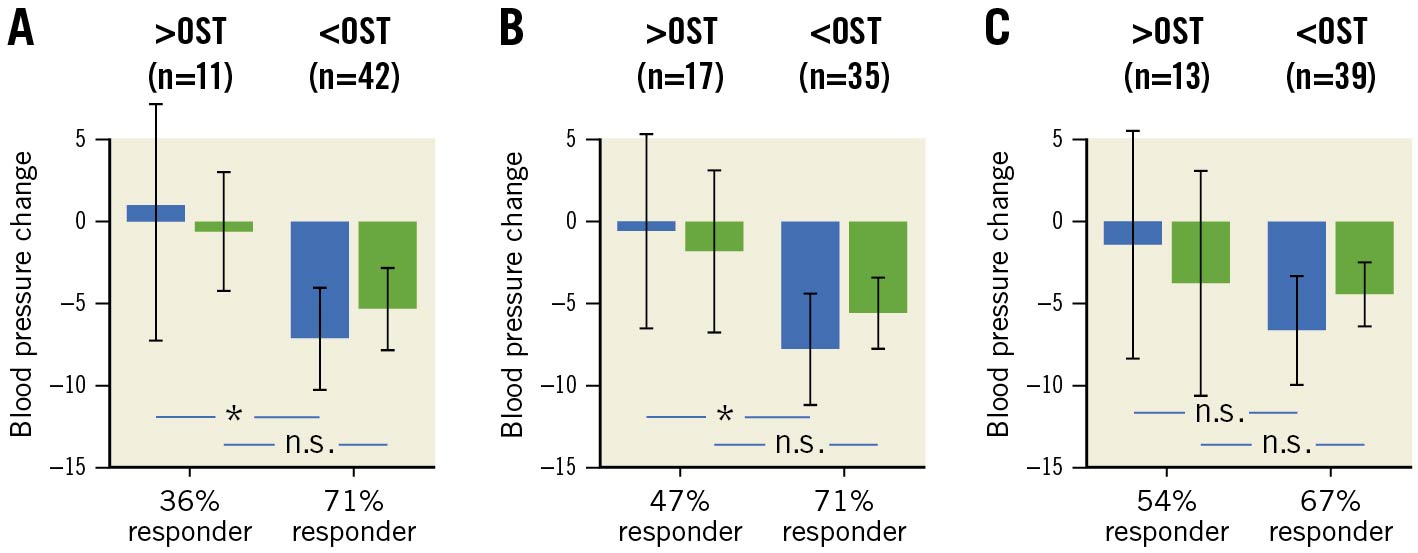
Figure 4. Validation of non-invasive prediction models – 24h systolic (blue bars) and diastolic (green bars) blood pressure change after 3 months, dichotomised by the optimal sensitivity threshold from the exploratory analysis for (A) ascending aortic distensibility, (B) the bivariable model and (C) the multivariable model. *p<0.05; n.s.: not significant. Error bars indicate 95% confidence intervals. OST: optimal sensitivity threshold
Application of a Youden index-based threshold instead of OST showed similar results, with significant BP difference between the groups for mAAD and the bivariable model and no significant difference for the multivariable model, but the group with a low likelihood for BP reduction at follow-up expanded to roughly 50% of patients with all 3 models.
Discussion
This trial is the first to prospectively confirm a predefined, independent predictor for BP reduction following RDN. Secondly, this is the first systematic prospective evaluation of a) clinical predictors, b) invasive, and c) non-invasive assessment of arterial stiffness as predictors for RDN success in a single trial. With these analyses, we were able to develop a validated, non-invasive prediction model for RDN success.
Our findings confirm the key role of arterial stiffening in the BP response to sympathetic nervous system modulation following endovascular RDN. Arterial stiffening has long been thought to be a major contributor to the endemic clinical problem of arterial hypertension16. Regarding RDN, it has been suggested as a potential treatment target17, but also as a predictor of RDN response. The latter is important, since an accurate and clinically applicable predictor of RDN response prior to the procedure is currently an unmet need.
As the accepted reference standard for arterial stiffness estimation, an elevated iPWV is usually associated with advanced vascular remodelling and loss of elastic cushioning function1318. It is also associated with increased pulse pressure and age1819. In contrast, renal norepinephrine spillover as a marker for renal sympathetic activity (the therapeutic target of RDN) is elevated in patients with essential and resistant hypertension2021 but seems to reduce with age22. Thus, in patients with exceedingly high iPWV, the contribution of sympathetic activity to BP elevation might be diminished. Instead, the contribution of biomechanical components to hypertension predominates, which makes BP reduction after RDN less likely. This concept is supported by our previous studies891011 and various other trials identifying different markers of vascular stiffening and ageing as negative predictors for BP reduction after RDN232425.
While accumulating data points towards iPWV as an independent predictor of RDN success, its invasive nature hinders its practical application in the general RDN population. We therefore investigated the potential of 2 non-invasive approaches to estimate arterial stiffness and consequently response to RDN: estimation of cPWV and an MRI-based model. Of all the markers assessed, only mAAD was independently associated with overall BP changes and confirmed in the validation study.
Contrasting our results on iPWV, using cPWV to identify patients with a low likelihood for BP change was futile. We also found a low correlation only between iPWV and cPWV. Besides measurement inaccuracies, this might be explained by the algorithm of the specific device, Complior, which tends to underestimate pulse wave velocity at higher levels of iPWV12. As mean and median iPWV were at very high levels in this study, the predictive accuracy of cPWV might have been hampered. Nevertheless, evaluation of other devices for cPWV estimation to predict BP change might be a worthwhile effort, as the use of a bedside tool could be more cost effective than MRI.
mPWV and mTAC also failed to precisely detect non-responders and to predict the extent of BP reduction at follow-up. This might be explained by the different methodology used for mPWV, which uses flow curves instead of pressure waves. Even though flow usually follows pressure increase, this could lead to a loss of precision in its accuracy when compared to iPWV. MTAC is only a very broad estimate of arterial stiffness and was numerically less precise than mAAD and iPWV in predicting BP change after RDN in one of our previous publications11. Altogether, both measures apparently lack the precision necessary to predict BP reduction after RDN.
The multivariable model was inaccurate in the validation cohort. This can be explained by a chance finding in our study cohort: improved BP reduction in women, and a lesser BP reduction in patients prescribed 5 or more antihypertensive drugs have never been described for RDN patients before, even in the large-scaled Global Symplicity Registry26. A device-specific factor for endovascular ultrasound RDN leading to this observation cannot be excluded here, but pathophysiologic assumptions make this explanation less likely. It might be an option to revalidate this model in an ultrasound RDN cohort in the future, but until then the multivariable model described here is not applicable in practice.
The bivariable mAAD-based model and logarithmic mAAD alone were the only non-invasive models predictive for RDN response, which remained significant after multivariable analysis and validation in a second study cohort. MAAD represents the central cushioning elements of the vasculature, which might give a more accurate estimation of vascular and biomechanical contribution to BP composition than iPWV, even though pulse wave velocity is regarded as the gold standard for arterial stiffness assessment13. In our previous study on prediction of BP response after RDN, mAAD was the only predictor with a numerically higher AUC than iPWV11, which is in line with the findings presented herein.
Even though MRI is cost-intensive, and its availability is limited, its use is often indicated or necessary in patients with treatment-resistant hypertension to exclude renovascular hypertension, as recommended by current guidelines, although this is usually a domain of duplex sonography127. It also facilitates RDN by identifying accessory arteries and potential access site-related pitfalls. In this setting, additional sequences to assess vascular stiffness before RDN could be performed with minimal extra cost and effort but yield important supplementary information.
Overall correlations between the prediction models and systolic BP changes might seem poor, but their practical implication is more significant: application of cut-off values for mAAD and the bivariable model leads to an exclusion of roughly 1/5 (mAAD) and 1/3 (bivariable) of patients with low pretest probability to RDN and only few potential BP responders among them. This allows minimisation of futile interventions in these patients who are the least likely to respond – an important advance in a population of resistant and refractory hypertensive patients, where prediction of BP response is often blurred by comorbidities and fluctuations in medication adherence328.
Of course, application of these models should not lead to general preclusion of patients with low pretest probability for success from RDN, but it could be a useful tool for shared decision-making between patients and practitioners and support a patient-preference centred treatment strategy.
The bivariable model is somewhat prone to regression to the mean, as it includes baseline systolic BP as a variable. Elevated baseline systolic BP repeatedly has been described as a strong predictor for RDN success82526. This could be explained by regression to the mean, but elevated BP could also represent a higher level of sympathetic activity and thus explain the improved responsiveness in these patients.
Limitations
Several limitations need to be mentioned. First, this is a single-centre trial in a highly specialised centre, which hinders generalisation of its results. Second, overall responder rates are lower than in recently published RDN trials234. This could be explained by a different trial population with more severe hypertension and intensified medical co-treatment. When compared to patients from the Global Symplicity Registry, who were on a similar level of antihypertensive treatment and had similar comorbidities, responder rates are similar26. Third, we cannot provide drug adherence testing for the patients enrolled. Poor drug adherence is a well-known problem in patients with treatment resistant hypertension, even in RDN trials328. Thus, results might differ when correcting for medication adherence or when conducting a trial in patients without antihypertensive drug treatment. Fourth, the validation cohort was small, consisted of patients with radiofrequency RDN treatment only and had a lower overall BP reduction than the study cohort. Even though this could also be interpreted in favour of the robustness of our findings for the bivariate model and mAAD, confirmation in larger scaled studies is warranted. Fifth, we did not include a control group in this trial. Thus, we cannot fully exclude that similar BP changes would occur without RDN spontaneously.
Conclusions
This trial confirms the predictive value of iPWV for BP reduction after RDN. It also supports the use of mAAD and a bivariable model, based on mAAD and baseline BP, as predictors for RDN response. The improvement in responder rates resulting from these models when applied to patients before RDN could facilitate trial design and change clinical decision-making in the future. Consequently, they could enhance the relevance of RDN as a treatment option in high-risk treatment-resistant hypertension patients in daily clinical practice.
Acknowledgements
The authors would like to thank Margit Büttner, Martin Petzold and Kai Trautmann for their support throughout the trial.
Impact on daily practice
For the first time in a prospective study, arterial stiffness is confirmed as a predictor of blood pressure reduction after renal denervation (RDN). Also, non-invasive prediction of this blood pressure response is feasible. Application of invasive and non-invasive prediction models for blood pressure outcome after interventional renal denervation could facilitate patient selection for future RDN trials as well as clinical decision-making.
Conflict of interest statement
P. Lurz and K. Fengler received modest institutional fees from ReCor Medical and Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

