
Abstract
Aims: The objective of the present study was to investigate an effect of renal artery sympathetic denervation (RASD) on patients with resistant hypertension and RASD effect on cardiac sympathetic nerve activity. It is known that an abnormally activated sympathetic tone is associated with progression of heart failure (HF).
Methods and results: We investigated 16 patients with resistant arterial hypertension (mean age 54.88±7.89 years, mean 24-hr ambulatory blood pressure [BP] systolic 161.07±20.12 mmHg, diastolic 97.6±16.25 mmHg, using 6.44±0.96 antihypertensive drugs), who underwent bilateral RASD. Echocardiography, 24-hr ambulatory BP and 123I-metaiodobenzylguanidine (123I-MIBG) scintigraphy were performed before and six months after RASD. There were no significant changes in 24-hr ambulatory systolic and diastolic BP before RASD and six months after it: systolic BP before RASD was 161.07±20.12 mmHg and 144.93±17.27 mmHg after (p=0.050); diastolic BP before RASD was 97.6±16.25 mmHg and 89.87±12.33 mmHg after (p=0.182). We observed a significant change in cardiac sympathetic nerve activity assessed by 123I-MIBG scintigraphy, as an increase of late heart-to-mediastinum (H/M) ratio, varying from 2.21±0.47 to 2.35±0.52 m/s (p=0.02).
Conclusions: Selective RASD significantly reduces cardiac sympathetic overdrive assessed by 123I-MIBG scintigraphy. Presumably, this positively affects HF progression in patients with resistant arterial hypertension.
Introduction
Hypertension is one of the most highly prevalent and most frequent chronic diseases worldwide. The prevalence of resistant hypertension, which is a major risk factor for myocardial infarction, stroke and mortality among adults, is increasing progressively1,2. Resistant hypertension is defined as a persistent elevation of blood pressure above goal, despite the concurrent use of at least three antihypertensive agents, each of a unique class, with a diuretic included among the treatment regimen, and with all drugs at target dose3,4. Even before clinical events occur, hypertension induces changes in the heart, including left ventricular hypertrophy (LVH) and cardiac fibrosis5. These are the mechanisms that play a major role in the development of HF. While numerous interventional treatment methods are being investigated, renal artery sympathetic denervation (RASD) is the one that has drawn most attention and is now widely used for the treatment of resistant arterial hypertension in hospitals across Europe. Activation of the sympathetic nervous system induces renal vasoconstriction and renin release, and increases sodium retention6. In addition, stimulation of afferent renal sympathetic nerves increases the overall sympathetic nervous system activity that plays a central role in the pathophysiology of BP elevation, as well as the development of LVH and heart failure5,7,8.
Several radiolabelled compounds have been used for non-invasive imaging of cardiac neuronal function. The norepinephrine analogue iodine-123 metaiodobenzylguanidine (123I-MIBG) is the tracer, most commonly used to map myocardial presynaptic sympathetic innervation and activity7. More recent data have suggested that RASD may result not only in decreased blood pressures, but also in a reduction of LVH9. Therefore, we used 123I-MIBG scintigraphy to evaluate whether this new therapeutic approach has a positive effect on decreasing myocardial sympathetic overdrive, which is an early sign of heart failure. 123I-MIBG scintigraphy shows changes that proceed at a cellular level. Therefore, we used this method to detect the smallest cellular alterations which show the earliest stage of HF. Our primary endpoint was to evaluate the effect of RASD on cardiac sympathetic nerve activity, assessed by 123I-MIBG scintigraphy, for patients with resistant hypertension. It is known that an abnormally activated sympathetic tone is associated with a progression of HF, and 123I-MIBG scintigraphy is likely to detect the earliest signs of HF at a cellular level. The secondary endpoints of the present study were to investigate RASD effect on office and 24-hr ambulatory blood pressure and arterial pulse changes in patients with resistant hypertension, as well as echocardiographic parameters before and six months after the RASD procedure.
Methods
The study was approved by the local ethics committee in accordance with the Declaration of Helsinki. All patients provided written informed consent.
STUDY SUBJECTS
Between March 2012 and February 2014, 147 patients (≥18 years of age) with suspected resistant hypertension were referred to a hypertension specialist in Vilnius University Hospital Santariskiu Klinikos. During the first visit, the patient’s current antihypertension medication plan was checked and changes were made, if applicable. One month after the treatment, corrected office and 24-hour ambulatory blood pressure measurements were performed. Patients were included in the study if they had an office systolic blood pressure (SBP) ≥160 mmHg (≥150 mmHg for type 2 diabetic patients), and/or mean 24-hr ambulatory systolic blood pressure of ≥140 mmHg, despite being treated with ≥3 antihypertensive drugs at maximum or with maximum tolerated doses (including one diuretic). Patients were included if they were not pregnant and had a glomerular filtration rate ≥45 mL/min/1.73 m2 (using the Modification of Diet in Renal Disease formula). These patients underwent a detailed examination using local protocol to rule out secondary hypertension, which included magnetic resonance imaging (MRI) of aorta, renal arteries and adrenal glands, or, in case of contraindications to MRI, computed tomography. Blood hormone testing included aldosterone, renin (aldosterone to renin ratio), metanephrine and normetanephrine. Patients with secondary causes of hypertension, as well as those who had type 1 diabetes mellitus, myocardial infarction, unstable angina pectoris, cerebrovascular accident within the last six months, or haemodynamically significant valvular disease were excluded from the trial. Patients with renal artery anatomy ineligible for treatment (haemodynamically or anatomically [main renal arteries <4 mm in diameter and <20 mm in length] significant renal artery abnormality or stenosis as well as a history of previous renal artery intervention) were also excluded from the study. After a detailed examination, 32 patients were eligible for RASD: five of them declined the intervention and 27 patients proceeded to RASD. Eleven patients declined cardiac 123I-MIBG scintigraphy. Therefore, the group which we analysed consisted of 16 patients with resistant arterial hypertension who underwent bilateral RASD and cardiac 123I-MIBG imaging (performed at baseline and after six months). All patients were investigated in stable cardiac status, without any changes in medications from baseline to month six. Cardiac MIBG imaging was performed under the same stable cardiac status, without changes in prescribed medications.
Clinical evaluation before RASD
HAEMODYNAMIC
All of the patients underwent a complete clinical history and physical examination, and review of medication. Patients were interviewed regarding whether they had taken their complete medication at the defined doses. Office BP readings were taken in a seated position after five minutes of rest according to the standard Joint National Committee VII guidelines10. BP and heart rate were recorded using an automatic oscillometric monitor on the brachial artery. Averages of the triplicate measurements were calculated and used for analysis. To exclude white coat hypertension, 24-hr BP recordings were performed in addition to office BP measurements.
ECHOCARDIOGRAPHY
All patients were examined using two-dimensional transthoracic echocardiography before the RASD procedure and at a six-month follow-up (Vivid 7; GE Healthcare, Horten, Norway). Images were acquired using a 1.5-4.6 MHz transducer in standard parasternal and apical two-, three-, and four-chamber views. Data were analysed and interpreted by two experienced echocardiologists blinded to clinical data and stage of the study. Analysis was performed to assess LV long-axis function by measuring mitral annular velocities with pulsed-wave Doppler tissue imaging according to the recommendations of the American Society of Echocardiography11 in the apical four-chamber view at the septal and lateral mitral annulus, with measurements averaged over three consecutive cardiac cycles. The following were also measured at rest according to the recommendations of the American Society of Echocardiography11,12: LV end-diastolic dimension, interventricular septal and posterior wall thickness, left atrial volume, mitral inflow velocities and deceleration time, and right ventricular systolic pressure.
LV internal dimensions and LV wall thicknesses were measured at end diastole and end systole12. According to these parameters, we calculated left ventricular mass (LVM) as follows: LVM (g)=1.04×(LV end-diastolic diameter+LV diastolic posterior wall thickness+LV diastolic posterior wall thickness) − (LV end-diastolic diameter)×13.613. The LVM index (LVMI) was obtained using the following formula: LVM/body surface area. LVMI was also derived by normalisation of LVM for height14. LVH was defined as LVMI >115 g/m2 for men and LVMI >95 g/m2 for women. Relative wall thickness was calculated as 2×LV diastolic posterior wall thickness/LV end-diastolic diameter, and was considered abnormal when >0.42. Relative wall thickness and LVMI were used to assess LV geometry15.
CARDIAC MIBG IMAGING
The evaluation of cardiac 123I-MIBG imaging was made without knowing the clinical data of patients’ cardiac status. Quantitative analysis of cardiac 123I-MIBG imaging was made by a single investigator.
Each patient was pre-treated with potassium iodine for thyroid blockade; later on, an intravenous injection of 210 MBq of 123I-MIBG was given. Fifteen minutes (early imaging) and four hours (late imaging) after MIBG administration, a two million count planar anterior image of the chest was acquired with a dual-head rotating gamma camera (Infinia; GE Medical Systems, Waukesha, WI, USA) equipped with a medium-energy collimator and stored in a 128×128 matrix. The MIBG activity was measured, using a manually drawn region of interest around the left ventricle (LV) and in the upper mediastinum. Mean counts per pixel were obtained in both the heart (H) and mediastinum (M). To assess MIBG myocardial uptake, the heart-to-mediastinum count (H/M) ratio was first determined from the early and late anterior planar MIBG images. The washout rate (WR) was calculated, using the following formula: ([early H - early M] – [delayed H – delayed M]×1.21)/(early H - early M)×100(%), where H=mean count per pixel in LV, M=mean count per pixel in the mediastinum. Cardiac MIBG scintigraphy was performed before RASD and six months after the procedure.
RENAL DENERVATION PROCEDURE
Renal denervation was performed by two experienced interventional cardiologists (>20 renal denervation procedures before the trial). After gaining femoral access, renal angiograms were acquired to confirm anatomic eligibility. The treatment catheter (Symplicity®; Ardian Inc., Palo Alto, CA, USA) was introduced into each renal artery using a guiding catheter. We performed up to six ablations in each renal artery, starting from the distal end of an artery and moving spirally to the ostium. Catheter tip impedance and temperature were constantly monitored, and radiofrequency energy delivery was regulated automatically by the generator according to a predetermined algorithm, with a maximum of 8 watts. In case of dual renal arteries or additional renal arteries, which were 4 mm or of a greater diameter, we proceeded with a standard ablation technique. We performed an average of 5.0±0.5 ablations in each renal artery and, in three cases of additional renal arteries, we performed four ablations in each additional artery. Periprocedural medications included heparin for anticoagulation and propofol for sedation. There were no periprocedural complications. After the procedure, all patients were treated with aspirin or clopidogrel for at least one month.
STATISTICAL ANALYSIS
The Wilcoxon matched-pair signed-rank test was used to evaluate changes between the pre- and post-treatment parameters. The Spearman correlation coefficient was used for a correlation analysis. STATISTICA software package (StatSoft Inc., Tulsa, OK, USA) was used for statistical analysis.
Results
Sixteen patients were included in the study, all of whom underwent RASD and cardiac 123I-MIBG scintigraphy at baseline and after six months. Baseline characteristics of the patients are shown in Table 1. Most patients were male (56.25%). The mean age was 54.9±7.9 years. The mean body mass index (BMI) was above normal at 34.16±4.02 kg/m2. On average, patients were taking 6.44 (±0.96) antihypertensive drugs. The most frequently used substance groups were beta-blockers (100%), angiotensin-converting enzyme (ACE) inhibitors (50%), angiotensin-1 receptor blockers (ARBs) (87.50%), calcium channel blockers (75.00%), aldosterone receptor blockers (62.50%), alpha receptor blockers (62.50%) and centrally acting sympatholytics (81.25%). All patients were on diuretic agents.
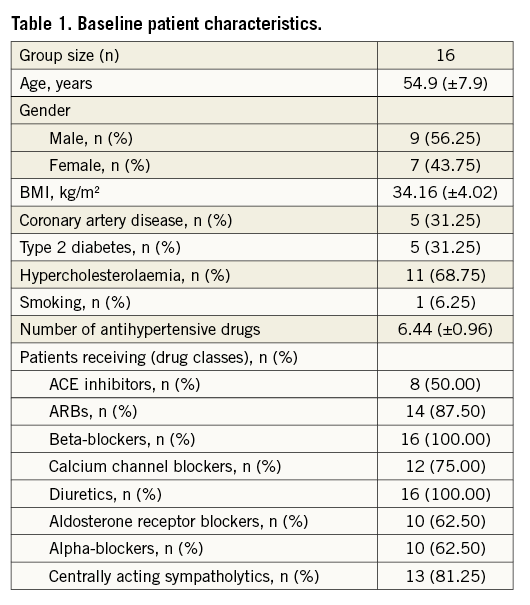
At baseline, overall mean sitting office systolic BP was 185.63±21.52 mmHg and mean sitting diastolic BP was 109.06±14.05 mmHg with a heart rate of 67.5±7.6 beats/min. Twenty-four-hr BP recordings showed mean systolic BP at 161.07±20.12 mmHg and diastolic BP at 97.6±16.25 mmHg with a heart rate of 64.47±7.87 beats/min. RASD significantly reduced sitting office BP at six months (systolic 152.69±14.88 mmHg, diastolic 90.31±10.2 mmHg, p<0.002), whereas 24-hr ambulatory BP remained unchanged (systolic 144.93±17.27 mmHg, diastolic 89.87±12.33 mmHg, p>0.05). BP changes are summarised in Table 2.
ECHOCARDIOGRAPHY
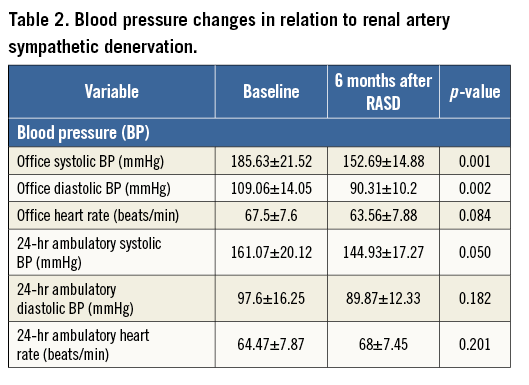
The echocardiographic parameters evaluated at baseline and during follow-up are summarised in Table 3. LV end-diastolic diameter remained unchanged (from 5.35±0.5 to 5.38±0.49 cm, p=NS), At six-month follow-up, interventricular septal diastolic wall thickness regressed from 1.32±0.27 to 1.15±0.18 cm (p<0.008) and LV diastolic posterior wall thickness from 1.21±0.22 to 1.06±0.11 cm (p<0.028). LVMI decreased from 136.18±39.42 to 112.65±22.79 g/m2. Concentric hypertrophy was the predominant LV geometric pattern and significantly improved at six months. Relative wall thickness decreased from 0.47 to 0.41 (p<0.025).
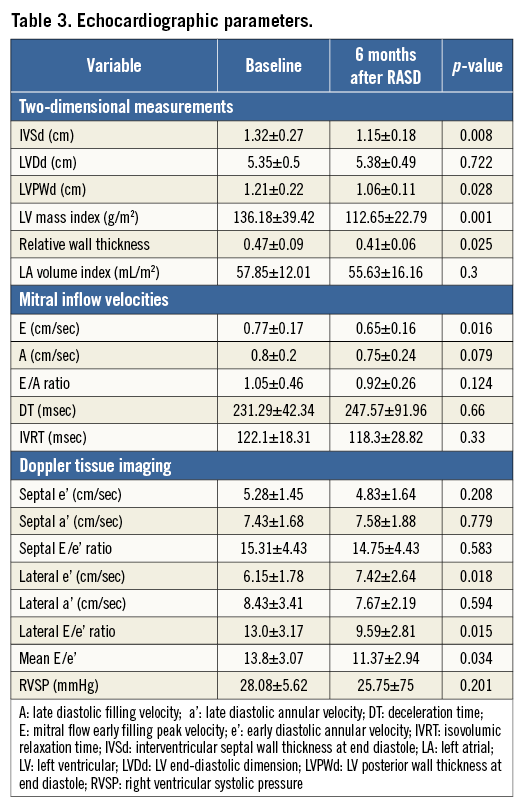
Patients who underwent RASD procedure showed significant concomitant improvements in LV diastolic function during follow-up. Six of 12 patients showed improvements in diastolic filling pattern after RASD procedure (Figure 1). Mean Lateral e′ significantly increased by 20.6% from 6.15±1.78 cm/sec at baseline to 7.42±2.64 cm/sec after RASD procedure (p<0.018). The mean E/e′ ratio decreased significantly by 17.6% from 13.8±3.07 to 11.37±2.94 after RASD procedure, indicating an improvement in LV filling pressure (p<0.034).
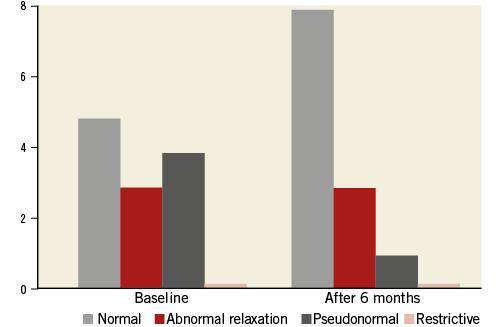
Figure 1. Diastolic dysfunction classification at baseline and after RASD procedure.
CARDIAC 123I-MIBG IMAGING
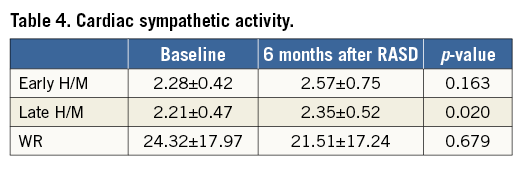
The baseline late 123I-MIBG heart/mediastinum (H/M) ratio was significantly lower, as compared with the H/M ratio six months after RASD (2.21±0.47 versus 2.35±0.52, p=0.020). There was no significant change in the early H/M ratio and WR for 123I-MIBG before RASD and six months after the procedure (Table 4).
Discussion
Resistant hypertension has been a focus of interest in the last few years because of RASD, which has been set forth as a solution to correct it16. Positive results were reported for patients with resistant hypertension who received RASD in the SYMPLICITY HTN-1 and SYMPLICITY HTN-2 studies4.
These studies were assembled into a large SYMPLICITY Registry16. However, in the SYMPLICITY HTN-3 trial the blood pressure decrease obtained in these strict conditions by RASD was not significantly different from the blood pressure decrease obtained from the best medical treatment16. There were many discussions about patient selection and quality of RASD which might have influenced outcomes in this trial. We think we should wait for the results of multiple trials before reaching conclusions about the use of RASD. The aim of our study was to look beyond the blood pressure lowering effect of RASD.
For many years, a reduction of peripheral BP per se was the target in the treatment of patients with arterial hypertension. However, increasing evidence indicates that the method of BP control may differentially affect the outcome. Mahfoud et al demonstrated that the renal sympathetic nervous system is an important regulator of insulin resistance and showed that RASD substantially improves insulin sensitivity and glucose metabolism, in addition to reducing BP significantly17. Brandt et al demonstrated a marked reduction of LV mass and improvement of cardiac functional parameters attained by RASD: reduced LV volumes, increased ejection fraction, and improved diastolic dysfunction, such as myocardial relaxation and end-diastolic pressures, as indicated by left ventricular mitral valve E/E’ and LA size5. LVH is an indicator of end-organ damage in arterial hypertension. The presence of LVH is associated with an increased rate of cardiovascular events and deaths independently of other cardiovascular risk factors and, notably, independently of BP values5,18-20. Despite the fact that we did not reach a statistically significant drop of 24-hr ambulatory BP after RASD, echocardiography showed important changes of cardiac morphology – reduction of LV mass and improvement of diastolic dysfunction.
Sympathetic overactivity is a key component of the signalling pathways altered in hypertension-related cardiac remodelling. Experimental evidence indicates that the sympathetic nervous system mediates hypertension-induced hypertrophy via direct stimulation of cardiomyocyte beta-adrenergic receptors5,21. Patients with heart failure show increased activation of the sympathetic nervous system, as reflected by an increase in plasma norepinephrine levels. Moreover, neuronal uptake of norepinephrine is impaired in the failing myocardium22. Most norepinephrine undergoes reuptake into presynaptic nerve terminals through the uptake 1 pathway.
Metaiodobenzylguanidine (MIBG) is an analogue of guanethidine that is taken up by the adrenergic nerve endings and shares common uptake (uptake 1 mechanism) and storage mechanisms with norepinephrine7,22. Its myocardial uptake depends on the integrity of the sympathetic pathway and correlates with the myocardial content of norepinephrine and the degree of myocyte necrosis22. Myocardial scintigraphy with 123I-MIBG provides images that reflect cardiac sympathetic nerve function. The uptake of 123I-MIBG is considered useful for the evaluation of the severity and prognosis of heart failure (HF), as well as response to treatment22-24. Some investigators have used 123I-MIBG imaging to assess cardiac sympathetic activity in HF patients undergoing cardiac resynchronisation therapy (CRT). Nishioka et al reported that the H/M ratio and washout rate were associated with CRT response, and that the H/M ratio was the only independent predictor of CRT response24. Similar results were obtained by our investigators group –Maneikiene et al– where early and late H/M ratios showed valuable prognostic power in predicting clinical outcomes of HF patients with wide QRS complexes undergoing CRT therapy25. Higuchi et al showed that CRT could improve cardiac sympathetic activity, as assessed by H/M ratio in patients with moderate to severe HF23. In our study, delayed H/M ratio was significantly higher six months after RASD procedure. As the delayed H/M represents the cardiac sympathetic overdrive, an increase of the delayed H/M ratio indicates a reduction of the sympathetic overdrive as a result of a successful RASD procedure. These data show improvement in biochemical HF markers that leads to a reduction of cardiac remodelling and mortality in patients with HF. Despite the fact that the second cardiac sympathetic innervation marked WR was lower six months after RASD procedure, the difference was not significant (p>0.05). These results could be due to the WR being a derivative dimension, where early H/M ratio, background and decay correction, and the early H/M ratio, representing cardiac sympathetic integrity, did not change significantly.
The aim of our study was to evaluate the changes in the cardiac sympathetic system due to treatment of resistant hypertension with RASD procedure, as a marker of good clinical outcome. The predictive value of cardiac 123I-MIBG imaging for RASD procedure success was not analysed due to the small patient sample.
Limitations
This study included a small number of patients in a single centre. Therefore, future studies of larger patient populations are necessary to determine the effect of RASD on cardiac sympathetic activity in HF patients.
Conclusions
RASD offers an innovative and safe catheter-based approach for selective reduction of renal sympathetic drive. We demonstrated that a selective denervation of the renal sympathetic nerves significantly reduces cardiac sympathetic overdrive as assessed by 123I-MIBG scintigraphy. This positively affects heart failure progression in patients with resistant arterial hypertension.
| Impact on daily practice RASD significantly reduces cardiac sympathetic overdrive assessed by 123I-MIBG scintigraphy and it positively affects heart failure progression. Our study shows that RASD has an additive value and might be effective in the treatment of end-organ damage in patients with resistant hypertension. |
Acknowledgements
This research is funded by the European Social Fund under the Global Grant measure.
Conflict of interest statement
The authors have no conflicts of interest to declare.

