Abstract
Renal sympathetic denervation has shown promise in treating hypertension resistant to drug therapy. This procedure lowers blood pressure via targeted attenuation of renal sympathetic tone. Up to now, it has also shown a favourable safety profile. Despite this, there is reason for cautious optimism, since longer follow-up is needed to address whether these effects may be considered definitive and to detect long-term side effects. To assess this, patients who have undergone this procedure should be submitted to a specific follow-up in centres of excellence in treating resistant hypertension.
Introduction
From 2000 up until now, catheter-based renal denervation has been tested in at least 25 clinical trials1-25 in patients with resistant hypertension (defined as systolic office blood pressure >160 mmHg –in diabetic patients >150 mmHg– treated with ≥3 antihypertensive drugs) generally resulting in a blood pressure (BP) decrease.
Enthusiasm for these results has been tempered by concerns about the durability of the effects of the procedure, the need for better understanding of the impact of renal denervation on a wide array of pathophysiologic cascades leading to hypertension, and the effect on renal haemodynamics. Indeed, antihypertensive efficacy has been reported to persist up to two years after the procedure12 with recent unpublished data suggesting efficacy up to three years26; however, longer follow-up is needed to address whether these effects may be considered definitive, since renal nerve fibres may regenerate27. However, the physiological relevance of anatomical regrowth of efferent nerve fibres in sustaining BP remains unclear28,29. Otherwise, no major adverse events have been identified so far; however, data on the long-term side effects are lacking.
Recommendation for the follow-up
As recommended by the European Society of Hypertension30 the assessment of outcomes and chronic safety at follow-up should be carried out in centres of excellence by experienced and specifically trained specialists, since post-procedural patient care is of particular relevance to maintain adherence to lifestyle factors and medications in order to achieve the maximum benefit from the procedure. Ideally, centre experience should be documented (Centre of Excellence of the European Society of Hypertension, European Hypertension Specialists). In Table 1, for instance, the UK Joint Societies consensus statement about selection of centres of excellence is summarised (Table 1)31.
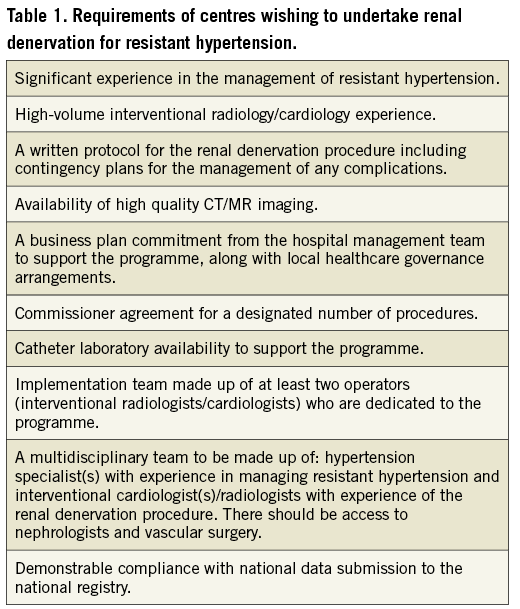
In addition, as currently recommended by position papers of different countries about renal denervation31-35, participation in national or international registries is mandatory, to obtain data on long-term efficacy and safety of the procedure.
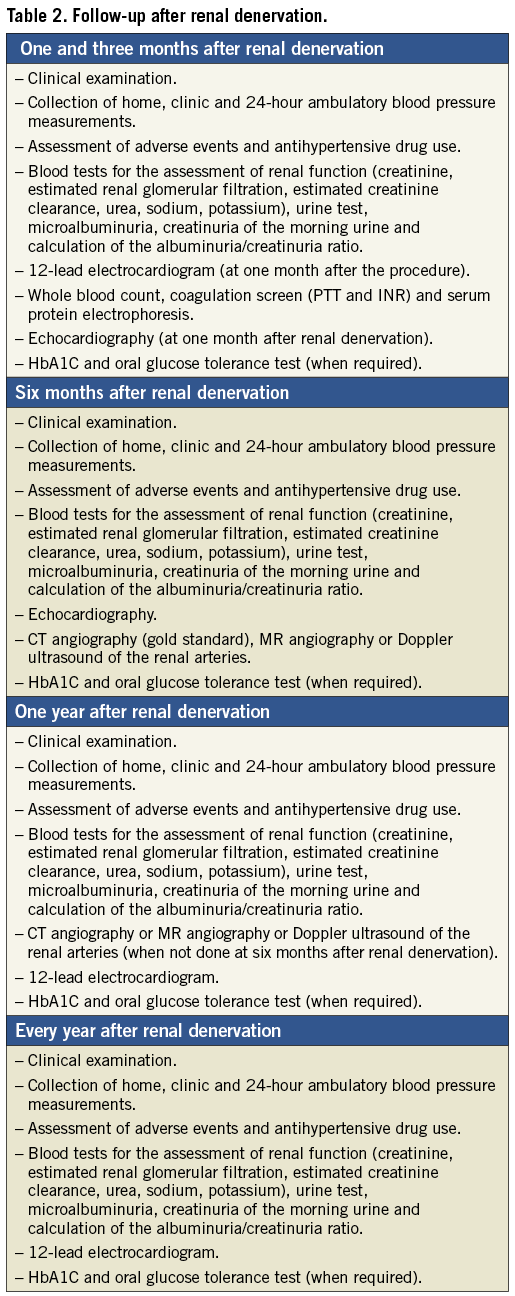
In Table 2, proposals about the timing of clinical examinations in the follow-up after renal denervation are illustrated. These are based on trials that have been conducted in recent years and from the recommendations of scientific societies recently published.
How to assess success of the procedure during follow-up
During the first year after the procedure, and especially during the first six months, clinical monitoring should be kept very tight32.
OFFICE BLOOD PRESSURE
Office BP should be recorded after ten minutes of supine rest both on the brachial artery at the first visit, and then on the same side throughout the follow-up. Averages of triplicate measurements with one to two-minute intervals should be recorded. A standard bladder (12-13 cm long and 35 cm wide) should be used, but a larger and a smaller bladder should be available for fat and thin arms, respectively. The cuff should be at heart level, whatever the position of the patient, and phase I and V (disappearance) Korotkoff sounds should be used to identify systolic BP (SBP) and diastolic BP (DBP), respectively.
The clinical effects of renal denervation could already be detectable one month after the procedure2,3,5,6,9,10,16, but it often takes several weeks to months before reaching peak effectiveness suggesting that a slowly progressive resetting of sympathetic neural regulation occurs4-6,9,10,16,19. Therefore, these deadlines are advisable to collect data of home BP and to check office BP, ambulatory blood pressure monitoring (ABPM) is also recommended.
AMBULATORY BLOOD PRESSURE MONITORING
There are conflicting results about the success of renal denervation in the light of the values of ABPM. Generally the effect is less pronounced, although in recent trials only small subgroups of patients have undergone ABPM before the procedure and at follow-up2-4,12. Among studies specifically designed to record effects of renal denervation on 24-hour BP, Prochnau10,16 reported a significant decrease in ABPM reading, while Vase did not. This may have been due to the limited number of patients and the lack of complete ablation achieved in two patients17. Witkowski and colleagues also did not find a significant decrease in the ABPM at three and six months, even though significant changes were reported in clinic measurement of BP15. Data regarding ABPM are relevant, since they provide further important information regarding the clinical effectiveness of renal denervation.
ANTIHYPERTENSIVE MEDICATION
One, three and six months after the procedure it is equally appropriate to assess the use of antihypertensive drugs. Indeed, despite cases of a decrease in the number of antihypertensive drugs required being mentioned in the literature4,17,35, the three- or six-month deadline has generally been used to assess the need for adjustment of antihypertensive therapy. Patients enrolled in clinical trials usually take approximately five antihypertensive drugs; at follow-up it has been observed that only in a small percentage of patients has a decrease in drug therapy been adopted, whereas a non-irrelevant percentage has run into an increased burden of drugs. Specifically, in Symplicity HTN-2 at six and twelve months after renal denervation 21% and 28% patients required a reduction in antihypertensive therapy, respectively, while 11.6% and 18.6% required an increase21. In three studies with a total of 236 patients, about 10% to 20% required a reduction in the number of medicines, while 10% to 25% required an increase4,5,12. Three studies with 129 patients reported a decrease in medications in 15% to 25% of the intervention group2,5,9,10. Ukena and colleagues reported a low rate of drug therapy reduction (5% in the intervention group)22. Other studies with a total of 60 patients reported no change in the number of medications1,14,16.
MONITORING ADVERSE EFFECTS AND SAFETY OF THE PROCEDURE
In the periprocedural period and immediately after, it is also important to detect possible adverse effects. In the literature, no long-term side effects have been published up until now among all patients treated worldwide. There have been two renal artery dissections during engagement with the guiding catheter, requiring stenting (without further complications)3,12. During the ablation, bradycardia has been observed in rare cases33. Minor complications included the formation of haematomas and pseudoaneurysms at the access site, urinary tract infection and loin pain2. Otherwise, it is necessary to monitor renal function with blood and urine tests (plasma creatinine, estimated glomerular filtration rate, cystatin C, microalbuminuria and macroalbuminuria). Although pathological consequences affecting renal function seemed to be generally excluded3,5,13-16,21, even in patients with renal insufficiency18,25, there is evidence that a percentage of subjects has developed a decrease in estimated glomerular filtration rate after the procedure12.
One month after the procedure it is also of interest to perform an electrocardiogram and an echocardiogram, since an improvement in indices of cardiac remodelling and ventricular diastolic function may already be detectable5. In addition, at three-month follow-up diabetic patients should be recommended to take a blood test for glycated haemoglobin and an oral glucose tolerance test4,15.
At the following control after six months, in addition to the above tests, it becomes necessary to perform a diagnostic imaging (Doppler ultrasound, angio-CT or angio-MR) of the renal arteries. In some cases, this investigation could be postponed or repeated a year after the procedure, while some protocols may also require an earlier one3,4. In most studies conducted so far, there were no vascular-renal complications attributable to the procedure itself. Two cases of progression of renal artery stenosis present at the time of the procedure are reported, in sites not subject to radiation, which required percutaneous stenting2,12. Recently, two cases of recurrent arterial hypertension have been reported five months after renal denervation due to the development of a renal artery stenosis, probably related to the procedure itself36,37. Indeed, the basis of this technology is an intended tissue injury caused by heat generated by radiofrequency energy that is limited to the renal sympathetic nerve fibres that are more sensitive to heat than the remainder of the surrounding tissue. Nevertheless, unintended tissue injury to surrounding vascular structures causing oedema and, perhaps, inflammation and fibrosis is conceivable. This might result in vessel stenosis. Despite this, a definitive statement cannot be made. However, the most likely aetiology of the renal artery stenosis in these cases was injury related to radiofrequency energy. Other possibilities include ablation catheter-induced mechanical injury and a de novo atherosclerotic lesion. However, both of these possibilities are less likely, given the absence of dissection by angiography immediately after ablation and mid location of one of the stenoses –a rather uncommon finding with atherosclerotic renal artery stenosis, because they are located predominantly at the ostium.
One year after renal sympathectomy, controls will become annual. Each time, the patient should undergo a complete clinical examination, data collection of home BP, side effects of the procedure and drug consumption, office blood pressure measurement, 24-hr ABPM, blood tests for kidney function, whole blood count and glucose metabolism (when necessary), electrocardiogram and cardiac ultrasound32,33.
Conclusion
Even if renal denervation has consistently shown significant post-procedure office BP reductions, sustained up to 24-36 months, in resistant hypertensive patients the experience with this procedure is still limited. The numbers of patients included in the studies are low and the duration of follow-up is short, which does not make it possible to evaluate the risk of rare and/or long-term adverse effects.
As future perspectives, new studies should be conducted in order to answer the questions that have yet to be resolved concerning renal denervation for the treatment of resistant hypertension. These studies should focus on such areas as the quantification of the decrease in BP using ABPM, criteria predictive of the efficacy of renal denervation via the endovascular route on BP, criteria of the efficacy of renal denervation during the procedure, BP efficacy and anatomical change in renal arteries after long-term follow-up and medico-economic evaluation of renal denervation via the endovascular route as a method of treatment for resistant hypertension. Obviously, future emerging indications for renal denervation would require different specific follow-up procedures.
Conflict of interest statement
The authors have no conflicts of interest to declare.

