Abstract
Percutaneous transcatheter renal sympathetic denervation (RDN) is a promising treatment for refractory hypertension (HT). RDN was found in one series of clinical studies to reduce systolic blood pressure (SBP) by as much as a mean of 30 mmHg with 85% of subjects experiencing sustained reductions of 10 mm or more out to two years after RDN. This degree of blood pressure reduction may reduce stroke and myocardial infarction rates and is anticipated to translate into improved life expectancy. The lowering of blood pressure by RDN has been shown to improve glycaemic control and reverse left ventricular hypertrophy . Beneficial effects on renal function, sleep apnoea and heart failure are suggested as well. This report describes the first patient treated using the OneShot™ Renal Denervation System (formerly Maya Medical now Covidien, Campbell, CA, USA).
Introduction
Percutaneous transcatheter renal sympathetic denervation (RDN) is a promising treatment for refractory hypertension (HT). RDN was found in one series of clinical studies to reduce office systolic blood pressure (SBP) by as much as a mean of 30 mmHg with 85% of subjects experiencing sustained reductions of 10 mm or more out to two years after RDN1,2. This degree of blood pressure reduction may reduce stroke and myocardial infarction rates and is anticipated to translate into improved life expectancy3. Lowering blood pressure by RDN has been shown to improve glycaemic control and reverse left ventricular hypertrophy4,5. Furthermore, beneficial effects on renal function, sleep apnoea and heart failure are suggested6-8.
The first-generation percutaneous device (Symplicity™; Medtronic, Santa Rosa, CA) achieves RDN by ablating sympathetic nerves that run in the renal artery adventitia by application of radiofrequency (RF) energy in a distal-to-proximal, point-by-point spiral pattern. This typically requires five or six ablations of two minutes duration to each renal artery.
The OneShot™ renal denervation system (formerly Maya Medical now Covidien, Campbell, CA, USA) used here is an irrigated radiofrequency balloon designed to deliver energy to achieve denervation of renal arteries using a single two-minute RF ablation to each renal artery.
Technical details
The major components of the OneShot™ system are the irrigated RF balloon catheter and a radio frequency generator (RFG) with an integrated pump. A mono-polar silver electrode is mounted on the non-compliant balloon in a helical configuration (Figure 1A) ensuring delivery of RF energy in a spiral pattern without the need to manipulate the catheter. The balloon is delivered to the renal artery over a conventional 0.014” interventional guidewire through a guide catheter. Radio-opaque markers identify the balloon ends for positioning under fluoroscopy. Balloon catheters are available in 5, 6 and 7 mm balloon diameter sizes and the balloon length is 20 mm.
The balloon is inflated to nominal size at 1 atm with normal saline delivered by the pump integrated to the RFG. The inflated balloon stabilises electrode contact with the renal arterial wall. During ablation, saline seeps from irrigation holes which are present along the electrode. These are designed to mitigate damage to non-target arterial tissue (Figure 1B). A single, two-minute, 25W RF ablation is delivered to each artery.
The RFG (Figure 1C) features a touch screen interface for user input, and displays instructional messages and procedural feedback such as power delivered, impedance, and remaining treatment time. The RFG displays warning messages and triggers automatic shut-offs to ensure safe operation of the system.
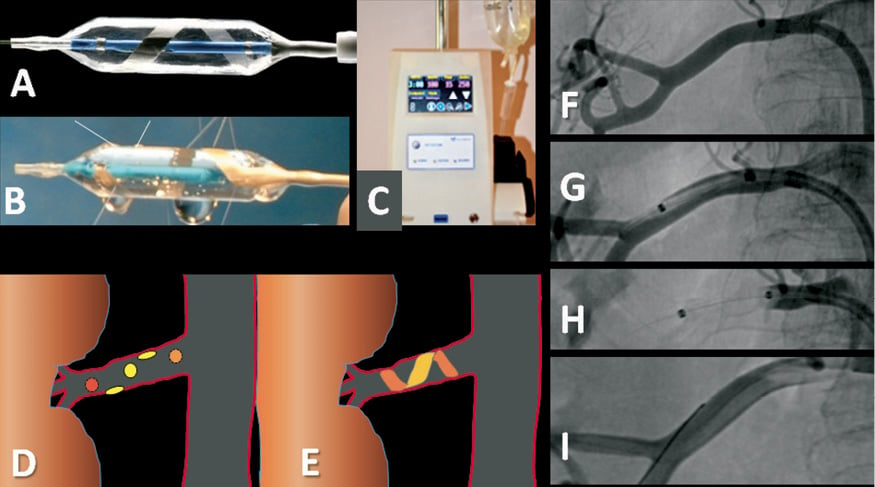
Figure 1. The Maya OneShot™ renal denervation system. A) shows the OneShot™ balloon catheter with the helical mono-polar silver electrode. B) Saline seeps from irrigation holes, cooling and protecting the non-treated arterial wall. C) OneShot™ RF generator with pump and touch-screen. D) Spiral point-by-point pattern desired for the multiple applications of RF energy from the Symplicity device. E) Single 2-minute spiral application of RF energy from the OneShot™ catheter. F) Right renal artery before treatment (postero-anterior projection). G) OneShot™ RF balloon identified by radio-opaque markers advanced over an 0.014” standard coronary guidewire into the renal artery. H) Inflated balloon apposing electrode to artery wall. I) Renal angiogram at procedure completion after intra-arterial nitroglycerine 200 mcg shows no visible injury to the artery.
Preclinical studies in a porcine model have revealed an excellent efficacy and safety profile at up to six months of follow-up. The lesions obtained were as expected with this mode of ablation. Renal norepinephrine content was markedly reduced and there was no instance of significant angiographic stenosis or intimal hyperplasia (data on file).
Case report
The patient was a 75-year-old non-smoking woman with impaired glucose tolerance and a long history of hypertension. Eighteen months earlier she had been admitted to the hospital with chest pain and a small rise in troponin levels. At that time, SBP was markedly elevated at >200 mmHg. Coronary angiography showed no significant coronary artery disease. No secondary cause of hypertension was identified. Despite multiple drug therapy (quinapril 40 mg twice daily, doxazosin 8 mg twice daily, metoprolol CR 95 mg daily, methyldopa 500 mg mane and 1 g nocte) her BP was poorly controlled. She reported recurrent headaches and general malaise and was intolerant of a number of antihypertensive medications, especially diuretics, which seemed to provoke postural hypotension leading to several syncopal events. Renal function was normal (eGFR >60 ml/min/1.73 m2 and creatinine 49 µmol/L). She gave written informed consent to participate in the Maya RHAS (Renal Hypertension Ablation System) Trial which had approval from the Auckland Ethics Committee.
A preliminary aortogram performed using a 6 Fr pigtail catheter introduced through a 9 Fr right femoral sheath identified the renal arteries and their take-off angles. A 9 Fr Vista Brite Tip® RDC guide catheter (Cordis, Miami Lakes, FL, USA) was used to engage selectively the right and left renal arteries. The diameter of the right renal artery was measured at between 4.5 and 5 mm (Figure 1F) by automated quantitative angiography (Philips, Eindhoven, The Netherlands). A 5 mm OneShot™ balloon was advanced over a 0.014” coronary guidewire (Figure 1G) and a single two-minute RF ablation was performed in the right renal artery (Figure 1H). Systolic BP rose from 195 mmHg following sheath insertion to 234 mmHg during ablation. A final angiogram demonstrated no damage to the renal artery (Figure 1I).
There were two left renal arteries, the more proximal of which was too small for treatment (diameter 3.4 mm). The more distal left renal artery measured 4 mm in diameter and was treated using the 5 mm OneShot™ balloon in the same manner as the right renal artery. The patient noted that the intensity of ablation pain declined (but did not disappear) about 30 seconds before the end of ablation on each side.
Activated coagulation time was maintained at over 250 seconds. For this first-in-human OneShot™ procedure, total fluoroscopy time was 7 minutes 28 seconds, overall procedural time 36 minutes, and contrast use 100 mL. The patient was pre-medicated with 100 mcg of fentanyl and 2 mg of midazolam. Further analgesia with 50 mcg of fentanyl was provided before each ablation.
At one-month follow-up her general wellbeing and headaches had improved. Office systolic blood pressure had fallen by 27 mmHg to 142, and mean 24-hour systolic BP by 16 mmHg to 153 mmHg (Table 1). At six-month follow-up, she remained well and her office systolic blood pressure was 141/64 mmHg on the same medical regimen as at baseline. Her renal function remained normal and a computed tomography angiogram of her renal arteries showed no abnormalities.
Conclusions
This first-in-human use of the OneShot™ renal artery denervation system successfully delivered RF energy to the renal arteries in a short and straightforward procedure. The reduction in blood pressure at one month was similar in magnitude to that seen in the Symplicity trials1,2.
In contrast to the Symplicity™ device, the operator does not have to redirect the ablation catheter for multiple point-by-point ablations (Figure 1D), hence delivery of RF energy should be more predictable and consistent with the OneShot™ catheter (Figure 1E). Additionally, the first-generation device requires five or six ablations on each side resulting in 24 minutes or more of ablation, whereas procedural time and duration of painful stimuli are both reduced using the balloon-mounted spiral electrode.
An 8 Fr/7 Fr-compatible system will shortly be available to replace the 9 Fr-compatible system.
Acknowledgements
Supported by the Auckland Heart Group Charitable Trust.
Conflict of interest statement
J. Ormiston is a minor shareholder in Covidien. The other authors report no conflicts of interest.

