Abstract
Out of the overall hypertensive population it is estimated that approximately 10% have treatment resistant hypertension (TRH). Percutaneous catheter-based transluminal renal ablation (renal denervation [RDN] by delivery of radiofrequency energy) has emerged as a new approach to achieve sustained blood pressure reduction in patients with TRH. This innovative interventional technique is now available across Europe for severe TRH for those patients in whom pharmacologic strategies and lifestyle changes have failed to control blood pressure below target (usually <140/90 mmHg). In 2012, the “ESH position paper: renal denervation – an interventional therapy of resistant hypertension” was published to facilitate a better understanding of the effectiveness, safety, limitation and unresolved issues. We have now updated this position paper since numerous studies have been published over the last year providing more data about the rationale, therapeutic efficacy and safety of RDN. In the upcoming ESH/ESC guidelines for the management of arterial hypertension, therapeutic options of treatment resistant hypertension will be addressed, but only briefly, and thus it is the focus of this paper to provide detailed and updated information on this innovative interventional technique.
Hypertension: a deadly disease
In the most recent comparative risk assessment of burden of disease, the leading risk factor for global disease burden was high blood pressure. In Europe, about 4.6 million people die every year of cardiovascular disease1 and, based on a worldwide analysis, 13.5% of the global mortality, 44% of stroke and 47% of ischaemic heart disease are causally related to arterial hypertension2. This situation will most likely worsen since the prevalence of hypertension is progressively increasing, and it is estimated that, by 2025, 29% of the world’s population will be affected3. It is not necessary that an increase of burden related to hypertension should occur as this can be attenuated since hypertension is a major modifiable risk factor for heart disease, stroke, end-stage renal failure and peripheral vascular disease. Indeed, at least in the USA, it is reported that according to the National Health and Nutrition Examination Survey 2001-2010, the use of antihypertensive medication increased from 63.5% to 77.3%, and the rate of treated hypertensive people, namely those whose blood pressure has been controlled, increased to 60% in 2009-20104. Of note, even modest blood pressure reduction led to a significant attenuation of cardiovascular morbidity and mortality, and this has been documented irrespective of starting blood pressure level5-8. Several appropriate and integrated pharmacological strategies are nowadays available, but blood pressure is still only poorly controlled in hypertensive patients and remains unacceptably high in Europe5,8,9.
Out of the overall hypertensive population, it is estimated that approximately 10% have treatment resistant hypertension (TRH)5,10. The prevalence of TRH varies according to population characteristics. TRH has been reported in 9% of all treated hypertensive patients among a general population living in the USA11. In a recent survey among more than 200 patients with hypertension, 1.9% developed TRH within 1.5 years (0.7 patient per 100 patient years follow-up)12. TRH is associated with a high risk of cardiovascular and renal events12. In a small analysis of nearly 500 subjects, patients with TRH showed a strikingly greater cardiovascular morbidity and mortality than controlled hypertensive patients13. Managing these patients with TRH presents an increasing clinical challenge to clinicians, and innovative antihypertensive strategies are eagerly awaited. Percutaneous catheter-based transluminal renal ablation (from now on referred to as renal denervation [RDN] by delivery of radiofrequency energy) has emerged as a new approach to achieve sustained blood pressure reduction in patients with TRH. This innovative interventional technique is now available across Europe for severe TRH in those patients in whom pharmacologic strategies and lifestyle changes have failed to control blood pressure below target blood pressure levels (usually <140/90 mmHg)14.
In May 2012, the “ESH position paper: renal denervation –an interventional therapy of resistant hypertension” was published to facilitate a better understanding of the effectiveness, safety, limitation and unresolved issues14. Over the last year numerous studies have been published, and we have now updated this position paper to reflect this, providing more data about the rationale, therapeutic efficacy and safety of RDN. In the upcoming ESH/ESC guidelines for the management of arterial hypertension therapeutic options of TRH will be addressed, but only briefly, and thus it is the focus of this paper to provide detailed and updated information of this innovative interventional technique.
Rationale of renal denervation
In a net-like pattern, renal efferent sympathetic and afferent sensory nerves run through the adventitia of the renal arteries15. Renal nerves contribute to the development and perpetuation of hypertension16 and efferent sympathetic stimulation results in sodium reabsorption, water retention, increased renin release and reduced renal blood flow secondary to renal vasoconstriction16. Less is known about the afferent sensory nerves, whose signals from the kidneys modulate central sympathetic outflow and thereby contribute to the neurogenic modulation of blood pressure. Renal afferent sensory nerves respond to stretch (mechanoreceptors), renal ischaemia, hypoxia or other injury (chemoreceptors) by increasing renal afferent activity to skeletal muscle circulation17-19.
Increased efferent sympathetic activity as (assessed by microneurography) has been uniformly shown in patients with primary hypertension, including “white-coat” and masked hypertension, pregnancy induced hypertension, secondary forms of hypertension (with the exception of hyperaldosteronism) and treatment resistant hypertension20-24. In particular, increased sympathetic activity to the heart and kidneys is strikingly elevated in arterial hypertension; and increases progressively in parallel with hypertensive stages being most elevated in patients with TRH.
Disrupting renal sympathetic nerves through surgical sympathectomy (subdiaphragmatic splanchnicectomy) has historically demonstrated good results in terms of blood pressure reduction and improved survival25. However, surgical sympathectomy causes serious side effects, such as postural hypotension, syncope, incontinence and impotence and was rapidly given up in the 1950s as newer pharmacological treatments emerged25. Nevertheless, recognition of the renal nerves as a potential target for treatment of hypertension has inspired clinical researches to develop an interventional approach to decrease the afferent sensory and efferent sympathetic signalling between the kidneys and the central nervous system. On April 8, 2003, a United States patent for “Renal nerves stimulation method and apparatus for treatment of patients” was filed and now, 10 years later, we have several clinical trials published on RDN in TRH.
To prove the concept in humans, single-unit and multi-unit muscle sympathetic nerve activity to skeletal muscles was recorded by microneurography before and after RDN to obtain information on the effect of removing the reflex sympathoexcitatory influence of afferent renal fibres. In a small study in 12 subjects (with normal and elevated blood pressure at baseline26), no significant change in multi-unit muscle sympathetic nerve activity was observed. However, in another study on 35 patients with TRH27, multi-unit muscle sympathetic nerve activity was moderately but significantly decreased and, more striking, all parameters of single unit muscle sympathetic nerve activity (including firing rates of individual vasoconstrictor fibres) were substantially reduced at three-month follow-up27. In the non-randomised control group, the sympathetic activity remained unchanged27. Furthermore, when global and renal noradrenaline spillover were measured before and after RDN, the overall and renal sympathetic activity were reduced after three months from the procedure by 19% (N=36) and 50% (N=19), respectively28,29. Thus, RDN causes a decrease of the overall sympathetic activity, presumably by removing the reflex influence of afferent renal drive. It definitively reduces to a marked degree sympathetic activity to the kidneys.
Technical issues
The ablation of the renal efferent sympathetic and afferent sensory nerves is performed with a radiofrequency catheter inserted percutaneously in the femoral artery. The catheter is (occasionally with the help of a guidewire) advanced in the lumen of the renal artery. The flexible tip of the catheter is placed distally before the first branch of the renal artery under fluoroscopic guidance. Good wall contact is mandatory to achieve sufficient heating of the adventitia in order to disrupt the renal nerve traffic fibres. This can be monitored by constant assessment of temperature and impedance at the tip of the catheter.
The procedure is performed on both sides, with at least four sides ablated in a longitudinal and rotational manner in two-minute treatments at each side in order to cover the full circumference. The duration of the procedure is typically 40-60 minutes. Since each radiofrequency ablation lasts two minutes with the single electrode system, a spiral multi-electrode catheter shortening the interventional time has been developed. Along these lines, other multi-electrode RDN catheters have been designed and are now tested in clinical trials. They use either a basket configuration at their distal end, constructed of four spines with one ablation electrode on each spine in a staggered position, a balloon-mounted radiofrequency ablation system or an ultrasound ablation catheter system. At present, five CE-marked RDN devices are available. The largest experience with longer follow-up exists only for the Medtronic single electrode tip catheter for energy delivery.
First clinical studies
The first proof-of-principle study was an international, non-randomised trial of 50 patients with TRH29. It was mainly a feasibility and safety study, and out of the 50 patients, five were excluded due to abnormal renal artery anatomy. Forty-five patients thus underwent RDN using the Symplicity Catheter system (Medtronic Inc., Minneapolis, MN, USA). Blood pressure changes and safety data were recorded. The Symplicity HTN-2 study was an open-label, randomised, multicentre study in 106 patients selected out of 190 patients with TRH30. The primary objective was to assess efficacy of RDN on office blood pressure after six months compared to a control group of medically treated patients. The inclusion and exclusion criteria in the two trials were very similar: office systolic blood pressure (average of three measurements) ≥160 mmHg (≥150 mmHg in diabetics), treatment with at least three antihypertensive medications including a diuretic, persistence of uncontrolled hypertension after a run in period of 15 days and absence of severe renal insufficiency (estimated GFR >45 ml/min/1.73 m2). In both Symplicity trials, the RDN intervention was not performed in 10-20% of cases due to anatomical reasons29,30.
Efficacy in Symplicity HTN-1 and -2
In Symplicity HTN-1 the baseline office blood pressure was 177/101 mmHg with 5.1 antihypertensive drugs on average. After bilateral RDN, office blood pressure was significantly reduced by –14/–10 mmHg, –21/–10 mmHg, –22/–11 mmHg, –24/–11 mmHg and –27/–17 mmHg at 1, 3, 6, 9, and 12 months, respectively. In nine patients medication was increased and in four patients decreased. In a small subset of patients renal noradrenaline spillover was reduced by 47%, documenting the effectiveness of sympathetic renal fibre ablation29. No 24-hour ambulatory blood pressure monitoring was applied.
In a larger group of similar patients, including the 45 patients of Symplicity HTN-1, the durability of blood pressure reduction was analysed during a longer follow-up31: 153 patients were included; mean age was 57 years and baseline office blood pressure 176/98 mmHg, with a mean of five antihypertensive medications. Office blood pressure after 12 months (N=130) was 23/11 mmHg, after 18 months (N=107) 26/14 mmHg and after 24 months (N=59) 32/14 mmHg31. Since then 36 month follow-up data have been presented, with the fall of blood pressure by 33/19 mmHg (N=24). Thus, substantial blood pressure reduction is sustained over three years of follow-up without any sign of rebound attenuation of the blood pressure efficacy after RDN.
In Symplicity HTN-2, a prospective randomised clinical trial, 106 subjects were randomised either to an immediate renal denervation (RDN group) or a delayed performance of the procedure (control group)30. Office blood pressure decreased in the RDN group by 32/12 mmHg (baseline 178/96 mmHg, with 5.2 antihypertensive medications), whereas no significant changes in the control group occurred (baseline blood pressure 178/98 mmHg, with 5.3 antihypertensive medications). When increases in medications were censored, the difference between the two groups was 31/11 mmHg. The percentage of patients with systolic blood pressure less than 140 mmHg after RDN was 39% (at baseline 0%) and between 140-159 mmHg 43% (at baseline 10%), thereby demonstrating a better blood pressure control after RDN. The percentage of responders, defined as a fall in blood systolic blood pressure by 10 mmHg, was 85% in the group treated by renal denervation and 35% in the medically treated control group30.
Recently, one-year results of Symplicity HTN-2 have become available32. Office systolic blood pressure at 12 months in the RDN group dropped –28 mmHg, which was similar to the six-month fall of –32 mmHg. Of the control group, 37 patients had RDN after six months and blood pressure decreased by –24 mmHg. In the extended follow-up to Symplicity HTN-1 and -2, blood pressure reduction has been confirmed as durable over 24 months in both groups (RDN group: –29/–10 mmHg [24 months], control group –28/–11 mmHg [18 months])33.
In summary, in the first proof-of-concept study and subsequent randomised prospective multicentre trials, RDN causes a substantial and significant fall in systolic and diastolic office blood pressure. This effect appears durable, without any sign of attenuated efficacy. Details of these studies are published elsewhere31,32.
Safety data
Overall, the Symplicity trials have demonstrated a good safety profile for RDN. In the proof-of-principle trial (Symplicity HTN-1) there were two procedural complications29: one, a renal artery dissection upon Symplicity catheter placement (before application of radiofrequency energy) and the other, the formation of a pseudoaneurysm at the femoral entry site. In the extended follow-up study of 153 patients (including the 45 patients of Symplicity HTN-1), two additional acute procedural complications (two pseudoaneurysms) occurred, all managed without further consequences31. Thus, four out of 153 patients had minor periprocedural complications. In the Symplicity HTN-2, no major serious complications were observed30. Throughout the procedure bradycardia was noted in 13% (N=7) of cases, some of them requiring atropine. Periprocedural side effects in the RDN group were: one femoral artery pseudoaneurysm (resolved with compression), one post-procedural drop in blood pressure requiring an immediate reduction in antihypertensive drugs, one urinary tract infection, one extended hospitalisation for assessment of paresthesias, and one case of back pain that was treated with analgesics and resolved after one month. In the cross-over group treated after six months, there was one renal artery dissection during guide catheter insertion before denervation, corrected by renal artery stenting, and one hypotensive episode which resolved with medical adjustment32.
In Symplicity HTN-1 and -2 renal function was carefully monitored and no significant change was found with respect to serum creatinine, estimated glomerular filtration rate and cystatin C levels after six months. Also, no adverse signal of renal function was observed in the longer follow-up period of Symplicity HTN-1 and Symplicity HTN-2. These data are consistent with other data that have now become available. In a series of 100 patients with RDN, no change in renal function was observed and, in another study, no change in renal perfusion (as assessed by an MRI spin labelling technique) was found after one day and one month34,35.
Renal vascular imaging after six months identified one patient with a possible progression of an underlying atherosclerotic lesion which required no therapy29,30,32. This abnormality was not near the location of energy application. In one patient, computed tomography (CT) angiography performed six months after the procedure revealed progression of an existing stenosis at the ostium of one of the renal arteries, which was successfully treated with stenting. However, the site of the stenosis was not in the area of energy delivery during RDN. In the extended Symplicity HTN-1 trial with 153 patients, follow-up imaging by duplex ultrasound or MRI at six months demonstrated an abnormality of the renal artery in one patient, with progression of a pre-existing renal artery stenosis31. This abnormality was described as being distant from the side of radiofrequency application. In a single case presentation, renal artery stenosis occurred after RDN leading to a reoccurrence of hypertension that was then treated successfully with angioplasty36. All these observations are incomplete, since no systematic imaging analysis by MRI or computer tomography has been carried out.
Ambulatory blood pressure after RDN
In 20 patients of the Symplicity HTN-2 trial, 24-hour ambulatory blood pressure measurements were conducted at baseline and after six months30. Mean reduction of systolic blood pressure was –12/–7 mmHg after RDN and no change in 25 controls. These data have to be interpreted in the context of an office blood pressure reduction –32/–12 mmHg and are limited to changes after six months. In a multicentre observational study, 303 subjects with true resistant TRH were followed after renal denervation and ambulatory blood pressure was obtained after 3, 6 and 12 months. Although yet unpublished, the change in office and ambulatory blood pressure after six months (–24/–10 mmHg and –10/–5 mmHg, respectively) was similar to that observed in the Symplicity HTN-2 trial37.
Such discrepancies in blood pressure response between office and ambulatory blood pressure values have not been described for interventional strategies only. In a meta-analysis of various studies analysing the antihypertensive efficacy of drugs, it was observed that the effect on 24-hour ambulatory blood pressure changes are generally smaller (about 60%) than that in office blood pressure38. In 409 patients with untreated hypertension, reductions in 24-hour ambulatory blood pressure were approximately 36% to 70% of the reduction by office pressure readings by the end of the trial39. Likewise, left ventricular mass can be reduced by smaller reductions in ambulatory blood pressure than much larger reductions in office blood pressure39. Thus, the strikingly smaller changes in ambulatory rather than in office blood pressure in Symplicity HTN-2 reflect a general phenomenon that is not restricted to interventional procedures. The “white-coat” effect of office measurements and the larger number of blood pressure readings available with ambulatory blood pressure monitoring (with the consequence of a narrower distribution of the values) may be two of the major underlying mechanisms contributing to explaining the different responses in office and ambulatory BP; although a placebo effect, and a regression to the mean phenomenon, both affecting only office and not ambulatory BP, may also play a role40.
Target organ damage
Of all the discussed intermediate endpoints of hypertensive disease, left ventricular mass, albuminuria and arterial stiffness parameters are considered the most valid and reliable intermediate endpoints of hypertensive target organ damage5,41. In 46 patients with TRH, left ventricular structure and function were assessed by echocardiography. Inclusion criteria of these patients was similar to Symplicity HTN-1 and -2 trials. In parallel to the reduction of systolic and diastolic office blood pressure (–28/–9 mmHg at six months), renal denervation reduced significantly septal and posterior wall thickness, decreased left ventricular mass from 54 to 45 g/m2.7 and improved systolic and diastolic functional parameters42. In two other studies, parameters of arterial stiffness and central haemodynamics improved significantly three and six months after RDN in patients with TRH34,43. Similarly, in a series of 100 consecutively enrolled patients with TRH, 88 underwent interventional RDN and 12 served as a control group. The renal resistive index decreased significantly at three and six months follow-up; at the same time the incidence of albuminuria decreased35. These studies indicate that, after RDN, intermediate endpoints of hypertensive target organ damage improved. Unresolved is the issue about whether these changes are related to the fall in blood pressure only or whether the reduction in sympathetic outflow of the central nervous system contributes substantially to this improvement in target organ damage. Moreover, it remains to be resolved whether blood pressure reduction due to renal denervation translates into improved cardiovascular prognosis and whether blood pressure reduction after RDN translates into cardiovascular protection to the same degree as observed in studies with pharmacologic drugs. The observed changes of the intermediate endpoints of cardiac, vascular and renal damage are quite reassuring, but confirmatory evidence needs to be established.
Patient selection and follow-up (Table 1)
Based on current evidence from available clinical studies, patients are eligible for RDN if they have severe treatment-resistant hypertension, defined by office systolic blood pressure ≥160 mmHg (≥150 mmHg in type-2 diabetes) despite treatment with at least three antihypertensive drugs of different types in adequate doses including one diuretic. These criteria have been used in nearly all clinical studies that have explored the effect of RDN on office blood pressure, ambulatory blood pressure and parameters of hypertensive target organ damage. Renal function as assessed by estimated GFR should be greater than 45 ml/min/1.73 m2, as done in the so far published clinical trials.
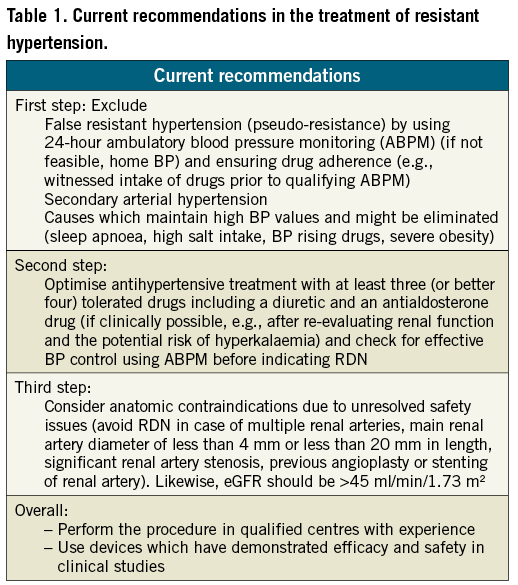
Going beyond these blood pressure criteria, i.e., offering RDN in clinical practice to patients with moderate TRH (office blood pressure between 140 and 160 mmHg, and 24-hour [daytime] ambulatory blood pressure monitoring >130 [135] mmHg) and to eGFR below 45 ml/min/1.73 m2 is a tempting possibility, but not at all supported by the current available evidence provided by clinical studies. These studies should be done first, prior to stretching the eligibility criteria outside of clinical studies.
Patients should have been evaluated by hypertension specialists in experienced hospital centres (e.g., ESH Excellence Centres, nationally approved RDN/HTN specialised centres) (Figure 1). For the first step, 24-hour ambulatory blood pressure monitoring or, if not available, home blood pressure measurements, should confirm sustained high blood pressure during real life conditions, thereby excluding pseudohypertension. False resistant hypertension has been found in up to 1/3 of treatment-resistant hypertensive patients44. Conversely, 24-hour ambulatory blood pressure monitoring may identify masked hypertensive patients45. After confirming a “true” resistant hypertension, secondary causes of hypertension should be re-evaluated and lifestyle and other factors that maintain high blood pressure should be corrected (obstructive sleep apnoea, high salt intake, blood pressure raising drugs). If these attempts are not successful within a limited time period, the uncontrolled hypertension status in these patients needs to be aggressively treated to prevent cardiovascular complications.
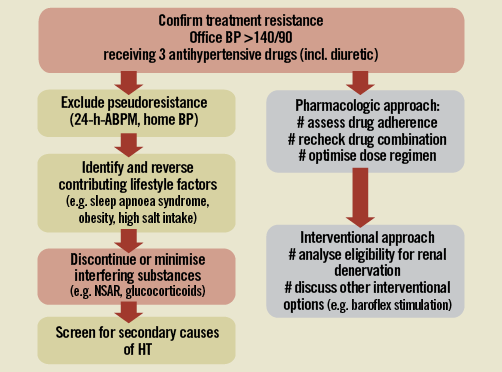
Figure 1. Diagnostic and treatment algorithm for the treatment resistant hypertension.
Particular attention is required to address non-adherence to drug therapy that is often present and not fully respected, if not rigorously investigated41. Incomplete adherence was found to be far more common than complete non-adherence and together represented the most common cause of poor blood pressure control in patients with TRH46. In clinical practice, non-adherence can be recognised by analysing the refill pattern of antihypertensive drug prescriptions, carefully interviewing the patient (and his/her relatives) and educating the patient about the relevance of hypertensive disease and its treatment.
Controversies exist across Europe about whether the use of aldosterone antagonists is a prerequisite for eligibility. Reductions of blood pressure by 22/10 mmHg in the ASCOT trial could not be repeated in the first, double-blind randomised trial with aldosterone antagonists in patients with treatment-resistant hypertension47. In face of a life-long therapy, the risk of hyperkalaemia associated with the use of aldosterone antagonists (e.g., spironolactone) is 4% in TRH47, but rises to 38.5% if chronic heart failure and eGFR <60 ml/min/1.73 m2 coexists48. In particular, unexpected decreases in eGFR due to developing comorbidities compromise the safety of aldosterone antagonists.
Patients claiming intolerance to some antihypertensive drugs may also represent candidates for RDN. However, the claimed intolerance to antihypertensive agents needs to be carefully analysed to determine whether it is really related to the administered compound and not to a patient’s psychiatric problems or unsuccessful physician-patient relationship. To diagnose true drug intolerance represents a challenge for hypertension specialists, since only true drug intolerance is a valid indication for interventional procedure such as RDN.
Certain anatomical situations represent a contraindication. The main renal artery diameter should not be less than 4 mm and the main arterial lengths should exceed 20 mm. In case of multiple renal arteries, the main branch should be treated if the anatomy is suitable. Previous renal artery interventions (balloon angioplasty or stenting), evidence of renal artery atherosclerosis (defined as renal artery stenosis >50%) represent current contraindications. Atherosclerotic lesions in renal arteries should be spared from radiofrequency energy delivery.
Patients should be in a stable clinical condition, since RDN is not an emergency treatment, thus ruling out patients with hypertensive crisis, cardiac complications or cerebrovascular evidence within the past 3-6 months. The procedure should be performed by an experienced interventional cardiologist, radiologist or angiologist who has a record of performing interventional procedures in the renal arteries and has been specifically trained for RDN. The centre should have the necessary infrastructure and qualified personnel to manage potential complications; e.g., dissection of the renal artery.
A cooperating network between general practitioners/family physicians, specialists and the experienced hypertension excellence centre is required for pre- as well as post-procedural care. Immediately after RDN, a patient’s blood pressure, heart rate and oxygen saturation should be carefully monitored in-hospital. Usually the duration of the hospital stay is between one and two days. Patients should have been trained to measure blood pressure at home, and office visits should be done after two weeks, four weeks and then in monthly, and later bi-monthly, intervals; or as frequently as the treating physician recommends in an individual patient. Antihypertensive treatments should not be discontinued immediately after RDN, because the expected decrease in blood pressure is delayed, and the efficacy of RDN can be judged only after six months.
Duplex ultrasound of the kidneys six months after RDN is optional, but monitoring of serum creatinine is mandatory. In the case of hypotensive episodes or symptoms, antihypertensive medication needs to be reduced on an individualised basis. Inclusion of hypertensive patients after RDN in national and/or international registries is desirable.
Limitation and unresolved issues (Table 2)
As of April 2013 only one randomised clinical trial has been published (Symplicity HTN-2). This study randomised 106 patients out of 190 patients screened for eligibility (since five patients had to be excluded due to abnormal anatomy), and this decreased further to only 45 patients. Clearly, a larger set of patients and randomised trials are urgently needed. The Symplicity HTN-3 study, currently recruiting and conducted in the USA, will close the gap as to what extent the fall in office blood pressure is related to a “white-coat” or/and placebo effect, since in this double-blind, randomised trial, a sham procedure is included in the control group. Fortunately, office and ambulatory blood pressure will be captured after six months and will provide further insight on the magnitude to which blood pressure reduction occurs after RDN.
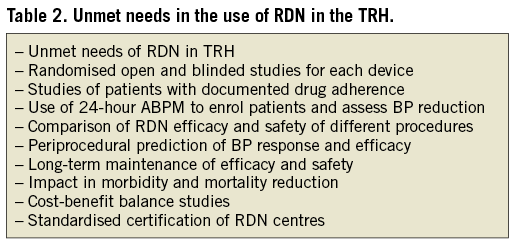
The duration of the antihypertensive effects after RDN needs to be further investigated since renal nerve fibres may regenerate49-51 and histological proof of re-innervation after RDN has now been experimentally demonstrated. Nevertheless, although nerve fibres may regrow, recovery of sympathetic function has not been demonstrated in humans. So far the longest reported durability of blood pressure decrease after RDN has been observed up to 36 months. Experimentally, after kidney transplantation, re-innervation has been found in rats, but functionally a state of persistent denervation has been observed51.
The lack of any procedural marker that might identify good responders to RDN is another matter to be addressed. At the moment, no direct assessment of efficacy during the procedure is available. Clinic determinants of responders have been analysed, but so far only pre-treatment blood pressure has been consistently found to predict blood pressure response. Other determinants such as age, gender, diabetic status, antihypertensive medication (patients on spironolactone, or on centrally sympathetic agents) have not been consistently found to predict blood pressure response. Additionally, up to now, there is no method to visualise or locate renal sympathetic nerves and thus to identify the target ablation site, although there are promising results from animal and first clinical studies using electrical intravascular stimulation52,53.
Safety is still a matter of concern and has to be thoroughly analysed separately for each individual CE- marketed device. Feasibility studies with 30 to 50 patients are not sufficient, and only large-scale registry or clinical trial data will have the power to discover low incidences of side effects. The Global Symplicity Registry is currently the only recruiting database that has been successfully set up, with the plan to include 5,000 patients followed for five years (current recruitment status >1,000 patients). Other CE-marked devices need to follow this line to obtain valid safety data.
Future direction of research
First reports have been presented that a higher number of radiofrequency ablations per side are associated with a greater blood pressure response, but these data are not consistently reported by all research groups. Whether pole arteries or accessory arteries need to be treated as well (under the assumption that these arteries also have a renal nerve fibre network along the adventitia) has not yet been properly addressed. Likewise, renal denervation of one side, with the other side left untouched due to anatomical or procedural contraindications, may also cause a substantial fall in blood pressure, most likely less than bilateral RDN, but no systematic analysis is available at the moment.
Reducing excessive central sympathetic activity is therapeutically attractive in the treatment not only of arterial hypertension, but also of several other diseases linked to sympathetic overactivity, such as chronic heart failure and chronic kidney disease. Thus, selective removal of renal afferent signals to the central sympathetic system appears an attractive treatment option to decrease the high morbidity and mortality rate in patients with chronic kidney disease and chronic heart failure. The first pilot studies have shown that functional parameters of congestive heart failure improved after RDN (six-minute walking distance54, ejection fraction, brain natriuretic peptide, clinical parameters55). In patients with chronic renal failure, RDN improved blood pressure control substantially, without having any harmful effects on renal function56. In patients on chronic haemodialysis, office and 24-hour ambulatory blood pressure improved in a small series of patients on chronic replacement therapy57,58. However, only randomised clinical trials will provide an answer to the question whether RDN is an additional therapeutic option in patients with chronic kidney and heart disease. Furthermore, elevated central sympathetic activity clusters with hypertension and insulin resistance, arrhythmia, sleep disorders, diuretic resistance and other diseases that may be addressed in the future53,59,60. It should be emphasised that in all future studies on RDN, office BP measurements should always be accompanied by out-of-office BP recordings through either home and/or ambulatory BP monitoring techniques.
Conflict of interest statement
R.E. Schmieder has received grants, lecture honoraria and consultancy fees from Medtronic Inc. J. Redon served as a lecturer for Boehringer Ingelheim, MSD, Daiichi Sankyo, Menarini, Takeda and was a member of advisory group for Daiichi Sankyo, Menarini, Takeda, Novartis. G. Grassi has received grants from Medtronic for the speaker’s bureau. S.E. Kjeldsen has received lecture honoraria from AZ, Bayer, Medtronic, MSD and Takeda, honoraria for consulting from Bayer, Medtronic, Takeda and Serodeus, and research support from AZ and Pronova. G. Mancia has received lecture honoraria from Bayer, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, Novartis Servier and Takeda. K. Narkiewicz has received lecture honoraria and consultancy fees from Medtronic Inc. G. Parati served as a lecturer for Daiichi Sankyo, Guidotti, Bayer and was a member of the advisory group for Daiichi Sankyo. L. Ruilope has served as advisor and speaker for Medtronic. P. van de Borne has served once as a speaker for Medtronic. C. Tsioufis has received a research grant by St. Jude Medical.

