
Few therapies for hypertension have had such a roller-coaster ride as renal artery denervation (RDN). Early optimism was enormous when trials reported reductions in systolic blood pressure (SBP) of >20 mmHg in patients resistant to standard pharmacotherapy. Yet euphoria turned to something close to despair when, later, rigorously designed trials failed to repeat the results. Recently, the therapy seems to have bounced back from its near-death experience. A series of trials have reported positive results and research has been revived. What are we to make of it all?
The unmet need is undisputed: hypertension is the most important cardiovascular risk factor in terms of its global incidence (around 1 billion people affected according to the World Health Organization [WHO]), with an astonishing prevalence of 35-45% of the general population. The incidence is predicted to increase with population growth and ageing. Despite advances in new pharmacological treatments and the widespread availability of combination pills, control of hypertension remains suboptimal.
It is no wonder that device-based treatments attract attention as alternatives to pharmacotherapy. The sheer size of the hypertension market is mouthwatering and highly investable for device manufacturers. For physicians and patients, the promise of relief from life-long drug regimens is very attractive. A reduction in cardiovascular outcomes is good for society as a whole.
The science underlying RDN is well established. It is well known that renal sympathetic nerves regulate renin secretion, tubular fluid reabsorption and renal haemodynamics, which modulates BP control. In the 1930s and 1950s it was observed that the surgical procedure of sympathectomy was associated with significant reductions in BP. Therefore, it was not surprising that, when initial unblinded studies of RDN showed a significant reduction in BP1, the results were met with enormous enthusiasm from clinicians and industry. Within months after the publication of these trials there were over 60 new start-ups in the RDN field.
After the boom, the bust. When the SYMPLICITY HTN-3 trial, the first prospective, masked, randomised study of RDN versus sham control, reported neutral outcomes2, there was general disappointment and disbelief. The fall-out was severe: many start-ups lost their investments and most of the multinational device giants stopped their RDN clinical trial programmes.
Extensive analyses were conducted post SYMPLICITY HTN to explore why the trial did not meet its primary efficacy endpoint. A number of confounding factors were identified, e.g., baseline SBP, use of and changes in hypertensive medications, adherence, study population, and procedural methods such as the number of ablation attempts, energy delivery and operator experience3. These confounding factors highlighted the complex and multifactorial nature of hypertension and indicated that addressing only one aspect of the disease might not be sufficient. The situation is further compounded by the fact that renal artery denervation is a blind procedure with no clinical or technical marker of successful nerve ablation during the procedure.
SYMPLICITY HTN-3 sent investigators and device manufacturers back to the drawing board, with regard to both technology and study design. An important new start is the SPYRAL HTN global clinical trial programme. It centres around the new Symplicity Spyral™ multi-electrode RDN catheter (Medtronic, Minneapolis, MN, USA). Trials have been designed to address a number of limitations in the earlier studies. The two initial trials focused on the effect of RDN in the absence (SPYRAL HTN-OFF MED) and presence (SPYRAL HTN-ON MED) of concomitant antihypertensive medications. Both were sham-controlled. Results were reported recently4,5 and received deserved attention.
In the proof-of-concept SPYRAL HTN-OFF MED, which included a three- to four-week drug washout period and a three-month follow-up period in the absence of antihypertensive medications, office and 24-hr ambulatory BP decreased significantly from baseline to three months in the RDN group: 24-hr SBP −5.5 mmHg, 24-hr DBP −4.8 mmHg; office SBP −10.0 mmHg, and office DBP −5.3 mmHg. No significant changes were seen in the sham-control group. This was already a world away from SYMPLICITY HTN-3.
SPYRAL HTN-ON MED was a large (n=467), multicentre study in which all patients were treated with a consistent triple therapy antihypertensive regimen. This trial too met its endpoints: there were significantly greater BP reductions in the renal denervation group than in the sham-control group at six months both for office BP (SBP difference –6.8 mmHg, 95% CI: –12.5 to –1.1; p=0.0205; DBP difference –3.5 mmHg, 95% CI: –7.0 to –0.0; p=0.0478) and for 24-hr ambulatory BP (SBP difference –7.4 mmHg, 95% CI: –12.5 to –2.3; p=0.0051; DBP difference –4.1 mmHg, 95% CI: –7.8 to –0.4; p=0.0292). These results were published in 2018 on the same day as a further sham-controlled RDN trial, RADIANCE-HTN SOLO, which investigated the ultrasound Paradise® renal denervation system (ReCor Medical, Palo Alto, CA, USA) in patients off antihypertensive medication6. The reduction in daytime ambulatory SBP in RADIANCE-HTN SOLO was greater with RDN (–8.5±9.3 mmHg) than with sham procedure (–2.2±10.0 mmHg). The baseline-adjusted difference between groups was –6.3 mmHg, 95% CI: –9.4 to –3.1; p=0.0001 in favour of RDN. Now we have three positive sham-controlled trials in a row, using different RDN technologies. So, what’s going on?
What earlier and recent clinical trials are telling us is that the identification of suitable patients and appropriate ablation locations is crucial for successful outcomes. There has been a shift in the thinking here. It is now believed that suitable patients are those with moderate hypertension and not resistant hypertension as initially thought. I believe that this can be attributed to the greater potential for moderating the underlying pathophysiology in moderate hypertensive patients compared with those with irreversible damage. Patients recruited in the SPYRAL OFF/ON trials were patients with moderate hypertension compared with patients with severe hypertension in SYMPLICITY HTN-3.
The second conceptual change is the location of denervation. In the SPYRAL OFF/ON trials, both main renal arteries and side branches were ablated, and the number of ablations was much higher than in the earlier, negative trials. However, it is interesting to note that in RADIANCE-HTN SOLO only the main branch was denervated, yet the reduction in office and 24-hr BP was similar in both off-medication trials. This may indicate efficacy differences between technologies: ultrasound achieves greater penetration of the renal artery than thermal energy (ultrasound 6-7 mm vs. radiofrequency 3-4 mm). We know that response to RDN is dose-dependent. Thermal energy might be equivalent to ultrasound in the main branch if the dose of thermal energy is equivalent to the dose of ultrasound.
A recently published prospective, randomised, single-blind, single-centre trial by Fengler et al7 supports these speculations, although in a different population. Patients with resistant hypertension were randomised in a 1:1:1 manner to either: 1) radiofrequency RDN of the main renal arteries, 2) radiofrequency RDN of the main renal arteries, side branches and accessories, or 3) endovascular ultrasound-based RDN of the main renal artery. At three months, there was a significantly greater reduction in systolic ambulatory blood pressure monitoring (ABPM) in the ultrasound ablation group than in the radiofrequency ablation group of the main renal artery (–13.2±13.7 vs. –6.5±10.3 mmHg; mean difference –6.7; 98.3% confidence interval –13.2 to -0.2; adjusted p=0.043). Radiofrequency ablation of the side branches achieved a non-significant additional –1.8 mmHg reduction over radiofrequency ablation of the main artery (98.3% confidence interval –8.5 to 4.9; adjusted p>0.99) and there was no significant difference between this group and the ultrasound group (mean difference –4.9 mmHg in favour of ultrasound; 98.3% confidence interval –11.5 to 1.7; adjusted p=0.22).
Although these comparisons are a good starting point, Fengler et al enrolled patients with resistant hypertension, did not have a sham group and the overall number of radiofrequency ablations in the side branch was smaller than the number of ablations in the SPYRAL-HTN studies. Clearly, large, multicentre head-to-head trials are needed to settle the issue and allow improvement in devices and procedures.
The greatest challenge currently is to identify responders to RDN. For this to be feasible, we will need safe, simple and reproducible technologies and markers to evaluate the extent of renal nerve ablation. Such technologies will permit assessment of procedural progress during the operation, allowing us to evaluate the effectiveness of the procedure, compare different technologies and compare different doses of therapies. In the current issue of EuroIntervention, Tsioufis et al report an evaluation of the ConfidenHT™ system (Pythagoras Medical Ltd, Herzliya, Israel) for diagnostic mapping of renal nerves8.
This initial feasibility study showed the ability of this system to stimulate renal nerves. This and other diagnostic tools might become very useful for identifying responders and might also provide a procedural marker of effectiveness.
The recent series of successful trials shows that RDN is back as a serious treatment alternative. We will probably never recover the initial euphoria and expectations. This is a good thing, if it means a more critical assessment of suitable patients and a push for continual improvement of ablation and diagnostic technologies. It seems increasingly clear that RDN deserves a place in the armoury of physicians dealing with hypertension. If the future roller coaster is less dizzying, we will all benefit. Figure 1 shows clinical, procedural and technical changes that may account for improvement from a negative trial for efficacy to positive trials for efficacy.
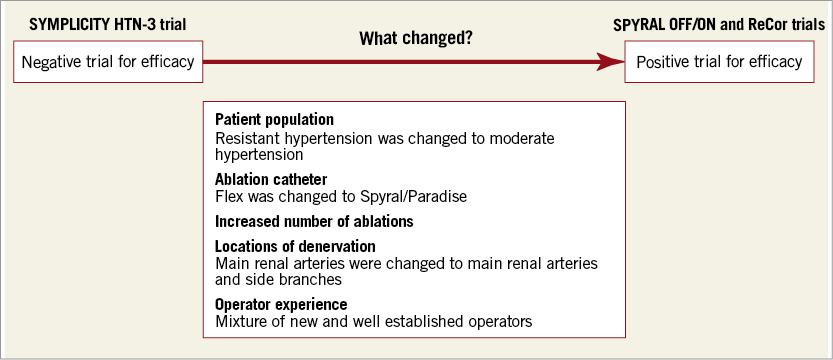
Figure 1. Clinical, procedural and technical changes that may account for improvement from a negative trial for efficacy to positive trials for efficacy.
Conflict of interest statement
The author has no conflicts of interest to declare.

