
Hypertension remains a major contributor to cardiovascular morbidity and mortality with increasing prevalence worldwide1. Despite the availability of several effective pharmacological treatment options, control of high blood pressure (BP) to target values remains challenging in many patients. Catheter-based renal denervation (RDN) is a treatment modality which targets renal sympathetic activity, a crucial component of the pathophysiology of uncontrolled hypertension2. With the publication of several recent randomised, sham-controlled trials3,4,5,6,7, the proof of principle for the blood pressure-lowering efficacy of radiofrequency and ultrasound RDN has been provided. In these studies, treatment with RDN when compared with sham was associated with larger falls in office and ambulatory BP. Hence, one may state that RDN is effective as an antihypertensive treatment - but is it also safe?
Acute or long-term injury of the treated vessel, vascular access-site and access-related complications are potential safety concerns for any device-based hypertension treatment8. In this issue of EuroIntervention, Townsend et al present a review and meta-analysis evaluating the incidence of renal artery damage after radiofrequency (RF) RDN using the Symplicity Flex™ or Spyral™ system (both Medtronic, Minneapolis, MN, USA)9.
A total of 50 published trials with 5,769 patients and 10,249 patient-years of net follow-up were reviewed for procedural safety. Twenty-six patients (0.45%) suffered an injury of the renal artery (0.33% stenosis, 0.12% dissection) with the necessity of stent implantation in 24 patients (0.42%). No cases of renal artery stenosis or dissection were reported after using the second-generation, over-the-wire multi-electrode, RF Spyral system. The median time from procedure to index event was 5.5 months and 79% of all events occurred within one year of the procedure. In a subset of 14 clinical trials (n=511), magnetic resonance imaging (MRI), computed tomography (CT) or angiography was performed. In this cohort, one new significant stenosis (0.20%) was identified after a median of 11 months post procedure.
The meta-analysis9 refers exclusively to RF RDN, clinically the most widely used technique. The findings are supported by the results of studies investigating other modalities of RDN, such as high-frequency ultrasound or chemical denervation with dehydrated alcohol5,7, though with a much smaller and limited sample size. In the RADIANCE-HTN SOLO trial, one out of 72 patients had a progression of pre-existing renal artery stenosis requiring revascularisation six months after ultrasound RDN. After an extended follow-up of 12 months (n=38), no de novo renal artery stenosis was detected by CT or MR angiography7. Also, after alcohol-mediated RDN in the Peregrine feasibility study (n=45), no relevant renal artery stenosis was detected after a follow-up of six months10.
Important performance metrics of device-based hypertension technologies, before their use can be recommended outside of clinical trials, are not only efficacy but above all safety. In this context, it may be relevant to review what has been learned about the safety profile of RDN in hypertensive patients.
Kidney function
The Global SYMPLICITY registry11 represents the largest cohort of hypertensive patients treated with RF RDN under real-world conditions (n=2,237). After a follow-up of three years, the estimated glomerular filtration rate (eGFR) was reduced by 7.1±16.7 mL/min/1.73 m2 in 289 patients with baseline eGFR >60 mL/min/1.73 m2 and by 3.7±16.2 mL/min/1.73 m2 in 93 patients with baseline eGFR <60 mL/min/1.73 m2. The reduction in eGFR corresponds to the expected decrease in patients with severe hypertension and could have been affected by lower perfusion pressure post procedure11. RDN with endovascular ultrasound did not result in relevant impairment of eGFR when compared with sham. At two months, there was no significant difference in eGFR change between the groups (adjusted mean difference –0.6 mL/min/1.73 m2, p=0·75)5. Following alcohol-mediated RDN, serum creatinine levels and eGFR remained unchanged during six months of follow-up10.
Response to exercise
Sympathetic nerve activity physiologically regulates heart rate (HR), cardiac output, BP, and ventilation, that could, theoretically, be impaired following RDN. The cardiorespiratory response during exercise has been investigated in patients with resistant hypertension undergoing RDN12. After three months, BP at maximum exercise was significantly reduced compared with baseline (21±20/5±14 mmHg, p<0.0001). However, there were no differences compared with the control group in maximum HR and HR increase during exercise as a sign of preserved chronotropic competence. Furthermore, work rate increased by 5±13 W (p=0.029) whereas peak oxygen uptake was not altered. One may conclude that RDN does not impair physiological response to activity.
Orthostatic function
The response to orthostatic alterations is another important function regulated by the autonomic nervous system. By reducing sympathetic activity and thus influencing vascular resistance, RDN could, in principle, induce orthostatic dysfunction13. Tilt table testing (TTT) is an effective tool to investigate orthostatic function under controlled settings14. In 36 patients with resistant hypertension, TTT with additional sublingual nitroglycerine provocation was performed before and three months after the procedure15. Mean or minimal systolic BP, especially in the early stage of orthostatic stress due to passive tilting and even after provocation by nitroglycerine, was not affected by RDN and the adaptive increase of HR was preserved.
Quality of life
As with other chronic diseases, resistant hypertension may adversely affect health-related quality of life (HrQoL). Multiple factors including knowledge of the disease, pharmacologically related adverse events and need for regular and frequent monitoring can cause reductions in HrQoL. The effect of RDN on QoL was investigated in 934 patients from the Global SYMPLICITY registry who completed the EuroQoL five-dimensions three-level questionnaire (EQ-5D-3L)16. At baseline, 32% of patients reported anxiety/depression and 48% reported pain/discomfort. Twelve months post procedure, a significant improvement in the overall perceived health state and anxiety/depression was observed. Another study investigated 119 resistant hypertensive patients and their psychological status, intensity of headache and stress tolerance six months after RDN. Patients reported significant improvements in QoL (p<0.05) and decreased levels of anxiety (p<0.0001), depression (p<0.0001) and intensity of headaches17.
In summary, RDN appears to represent not only an efficacious but also a safe treatment option for patients with uncontrolled hypertension (Figure 1). The meta-analysis by Townsend et al9 provides reassurance concerning the vascular safety of RF RDN. The primary guiding rule when selecting the appropriate patient population for device-based hypertension treatment approaches is that the risks associated with the procedure should not exceed the underlying risk of the untreated condition for each individual patient, especially when used in milder forms of hypertension with lower risk for cardiovascular complications8. Longer-term safety in larger cohorts of patients with rigorous and complete follow-up is required for final assessment of the safety profile of current RDN devices. We have not reached the finish line yet, but we are well on our way to achieving it.
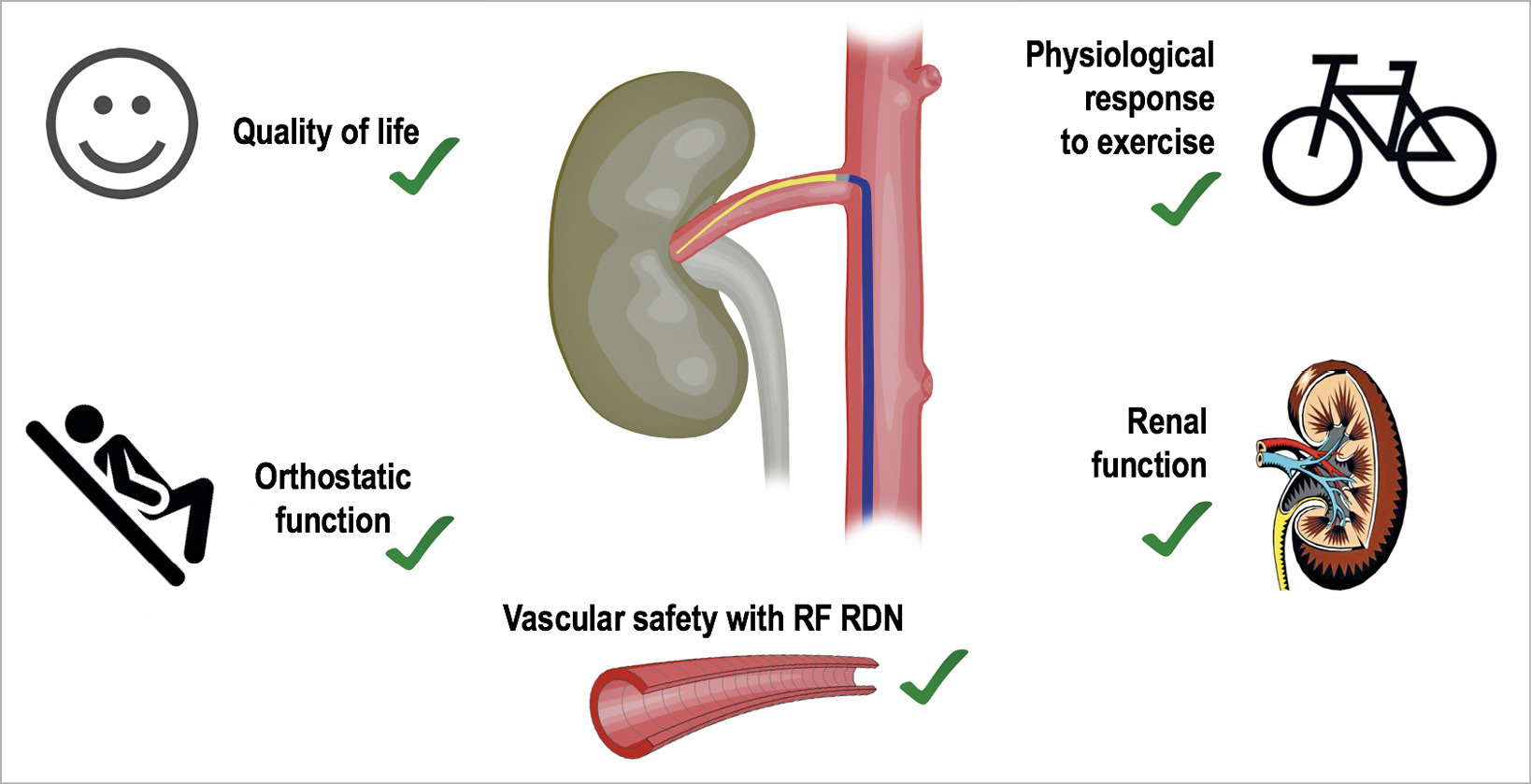
Figure 1. Safety profile of renal denervation. RDN: renal denervation; RF: radiofrequency
Conflict of interest statement
F. Mahfoud is supported by Deutsche Gesellschaft für Kardiologie and Deutsche Forschungsgemeinschaft (TRR SFB- 219) and has received speaker honoraria from Medtronic, ReCor, Bayer, and Boehringer Ingelheim. The other author has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

