
Introduction to the session
The aim of this summary report is to capture the content of the session at EuroPCR 2018 that reviewed the implications for clinical practice of the SPYRAL HTN-ON MED study1, in order to share the critical analysis of the trial and report the views expressed in the interactive discussion. This article does not constitute an independent review of the topic by the authors.
What was known before SPYRAL HTN-ON MED?
Hypertension is highly prevalent around the world and is associated with cardiovascular morbidity and death. However, blood pressure (BP) control remains unacceptably low and the number of patients with uncontrolled hypertension will increase significantly, considering the revised lower target BP values which have been suggested by the recently published US report on the management of hypertension2. Catheter-based renal denervation (RDN) is under investigation as a minimally invasive treatment option for patients with uncontrolled hypertension3. Several studies and registries have confirmed its BP-lowering effectiveness4. Importantly, the randomised controlled DENERHTN study showed superiority of RDN in combination with optimal and standardised antihypertensive medication compared to pharmacotherapy alone in terms of ambulatory BP-lowering effect at six months5. In contrast, the randomised, sham-controlled SYMPLICITY HTN-3 trial showed no significant difference in BP reduction between patients in the RDN or sham group after six months6. In brief, several potential confounders have been identified which have informed the design of a new set of clinical studies. These confounders include inadequate patient selection, low operator experience, insufficient procedural performance, and uncontrolled changes in antihypertensive medications by healthcare providers and variable adherence to treatment by the patients7.
The SPYRAL HTN-OFF MED trial was one of the sham-controlled trials designed subsequently to avoid these methodological pitfalls. The trial included patients with mild-to-moderate hypertension not treated with antihypertensive medications. An average of 44 ablations including an average of 26 branch ablations was performed using the Symplicity Spyral™ multielectrode radiofrequency catheter (Medtronic, Minneapolis, MN, USA). All cases were performed by experienced proceduralists and proctored using detailed treatment plans. The first results were derived from an interim analysis at three months and included 80 patients randomised to RDN (N=38) or sham procedure (N=42). The key efficacy endpoint, a significant between-group difference in 24-hr ambulatory and office BP change in favour of RDN, was met8. The trial provided biological proof-of-principle for the BP-lowering efficacy of catheter-based RDN in patients with mild-to-moderate hypertension not treated with antihypertensive medications8.
What is SPYRAL HTN-ON MED about?
The prospective, randomised, sham-controlled SPYRAL HTN-ON MED study (NCT02439775)1 investigated the BP-lowering efficacy of radiofrequency-based RDN in combination with pharmacotherapy. A total of 80 patients (N=38 RDN; N=42 sham control) were randomised at 25 centres in the USA, Germany, Japan, UK, Australia, Austria and Greece and followed up to six months. Eligible patients were 20-80 years old and had uncontrolled hypertension (office systolic BP 150-180 mmHg, office diastolic BP ≥90 mmHg and 24-hr ambulatory BP of 140-170 mmHg) despite prescription of one to three antihypertensive drugs with stable doses for at least six weeks. An average of 45.9±13.7 ablations including an average of 26.6±11.7 branch ablations was performed using the Symplicity Spyral multielectrode catheter. All cases were performed by experienced proceduralists and proctored using detailed treatment plans. The first results were derived from an interim analysis at three months and six months. Office and 24-hr ambulatory BP were significantly reduced from baseline to six months in the RDN group and the change in BP was significantly greater at six months in the RDN group than in the sham-control group despite antihypertensive treatment (Figure 1). However, adherence to antihypertensive treatment (assessed by blood and urine testing with patient awareness) was moderate in both groups. Further, BP progressively decreased during follow-up: the changes in 24-hr ambulatory systolic BP at three months were –0.7 mmHg (sham control) versus –4.3 mmHg (RDN group) and at six months –1.8 mmHg (sham control) versus –8.8 mmHg (RDN group). Ambulatory blood pressure analysis over 24 hours also showed a consistent reduction in blood pressure at all time points compared with baseline in the RDN cohort versus no change in the sham-control group. This “always-on” effect of RDN distinguishes this therapy from the pharmacokinetic limitations of medical therapy as well as patient non-adherence. Notably, there were no treatment-related adverse events in either of the study arms during six-month follow-up.
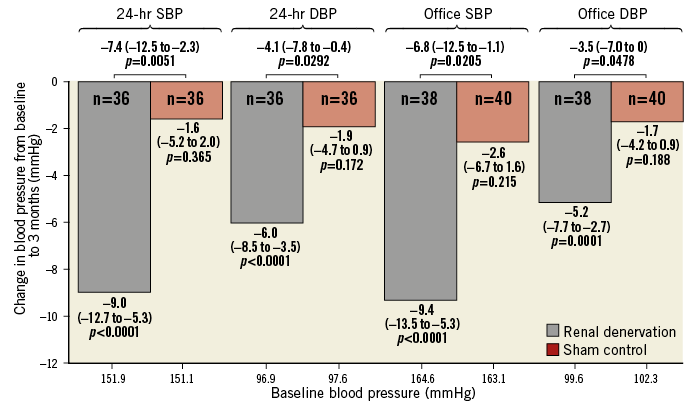
Figure 1. Change in office and ambulatory systolic blood pressure and diastolic blood pressure for treatment and sham-control patients at six months. Data are mean (95% CI). DBP: diastolic blood pressure; SBP: systolic blood pressure. Modified with permission from Kandzari et al1.
Will SPYRAL HTN-ON MED change my clinical practice?
After a lively discussion, the participants agreed that the data of the SYPRAL HTN-ON MED study presented at EuroPCR 2018 are clinically important as in this trial patients who have been prescribed up to three antihypertensive agents were investigated. This cohort represents the vast majority of hypertensive patients. The results add to the growing body of evidence, such as the DENERHTN5, SPYRAL HTN-OFF MED8 and RADIANCE SOLO9 trials, indicating the continued safety and efficacy of RDN with the different RDN systems (RF or ultrasound ablation) in both the presence and absence of antihypertensive medication after two to six months.
In the SPYRAL HTN-ON MED trial, RDN reduced 24-hr ambulatory systolic BP by 9.0 mmHg (p<0.001) and office systolic BP by 9.4 mmHg (p<0.001) after six months. Discussions during the session covered the clinical meaning of these findings. It is indeed essential to reiterate the importance of BP lowering to reduce major cardiovascular endpoints and mortality. Based on a meta-analysis including more than 600,000 patients, an office BP reduction of 10 mmHg is associated with a relative risk reduction of coronary heart diseases by 17%, stroke by 27%, heart failure by 27%, and mortality by 13%10. However, whether the BP-lowering effect observed following RDN also improves cardiovascular outcome (if maintained in the long term) has not been proven as yet.
Interestingly, the ambulatory BP reduction continued after RDN over the follow-up period and was more pronounced after six months (–8.8/–6.1 mmHg) than after three months (–4.3/–4.2 mmHg). The underlying mechanisms of this progressive BP change over time are incompletely understood and may include central sympathetic nervous resetting, alterations in the renin-angiotensin-system activity and reverse vascular remodelling. The available evidence does not support the existence of post-procedural reinnervation of the sympathetic renal fibres up to six months; however, long-term data will be needed to address this important issue.
Although antihypertensive drugs are generally well tolerated, almost one third of all hypertensive patients do not initiate a new prescription of antihypertensive drugs11 and around 50% of them become non-adherent in the first year after initiation of antihypertensive treatment12. In the SPYRAL HTN-ON MED trial, medication adherence was tested using tandem high-performance liquid chromatography and mass spectroscopy of urine and plasma at baseline, three, and six months. Despite the fact that patients were aware of the toxicological assessments, about 40% of them were non-adherent to all antihypertensive drugs with no difference between groups at all time points1. This is in line with other studies in the field of device-based hypertension treatment13 and underlines the major issue of non-adherence to oral medications in hypertension, as in other chronic diseases12,14.
Twenty-four-hour ambulatory BP measurements documented a sustained BP reduction throughout the day and night, thus indicating an “always-on” treatment effect after RDN, which was distinctive from short-acting pharmacotherapy, where the effect subsided between doses (Figure 2). These data suggest that RDN provides an additional BP-lowering effect that is complementary to antihypertensive drugs and offers the possibility of constantly reducing BP, even in non-adherent patients. However, this trial cannot answer the question of which antihypertensive agents are optimal for concomitant therapy, and it remains important to investigate further the interaction of antihypertensive medication and RDN.
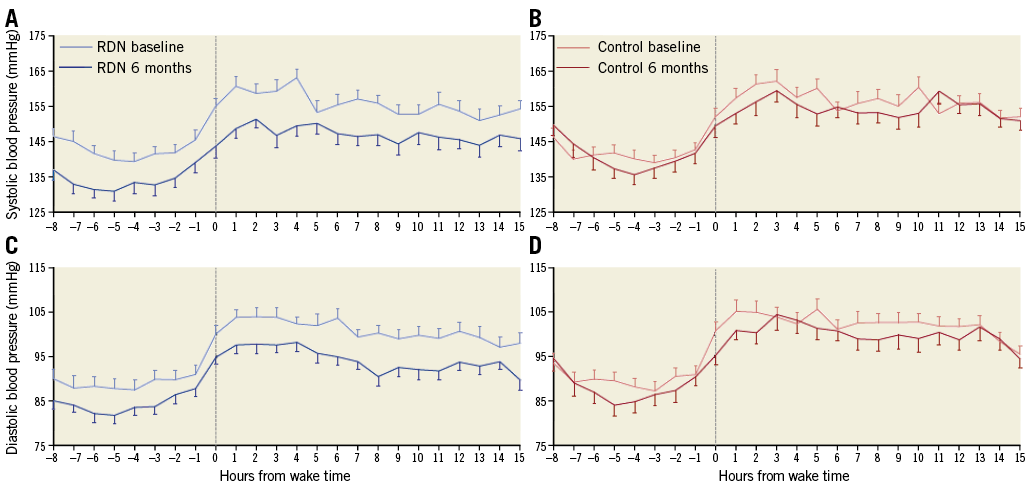
Figure 2. Hourly ambulatory systolic blood pressure and diastolic blood pressure at baseline and six months, according to patient-recorded individual wake times. Time zero represents patient wake time or 07:00 for patients not reporting; error bars represent the standard error. A) 24-hr ambulatory systolic blood pressure at baseline and six months for renal denervation group. B) 24-hr ambulatory systolic blood pressure at baseline and six months for sham-control group. C) 24-hr ambulatory diastolic blood pressure at baseline and six months for renal denervation group. D) 24-hr ambulatory diastolic blood pressure at baseline and six months for sham-control group. Modified with permission from Kandzari et al1.
Unfortunately, there is still no technology available to predict the direct or long-term response to RDN. The available data cannot provide a conclusive explanation for the large post-procedural variance. Of central importance remains the question which patient population benefits in the long term from the minimally invasive procedure. In SPYRAL HTN-ON MED, only patients with a combined hypertension (>140/>90 mmHg) were included. Although RDN has shown reductions in office and ambulatory BP in patients with isolated systolic hypertension (>140/<90 mmHg), the reduction is more pronounced in patients with combined systolic-diastolic hypertension15,16.
SPYRAL HTN-ON MED prompts further trials to investigate long-term efficacy and safety as well as RDN as a treatment option in other conditions associated with sympathetic overdrive, such as chronic kidney disease, chronic heart failure and atrial fibrillation. The U.S. Food and Drug Administration has already approved the SPYRAL HTN-OFF MED Pivotal Trial (NCT02439749), which will further enhance our knowledge about the potential role of RDN in the armamentarium of antihypertensive treatment approaches.
The Chairperson’s conclusion: where do we stand now?
In summary, in spite of numerous years of effort to control elevated BP through lifestyle modifications and medications, many patients remain off BP targets. New approaches are critical and necessary in order to increase the control rates and reduce patient risk. Stay tuned, progress exists - the work continues.
Conflict of interest statement
S. Ewen and F. Mahfoud have received scientific support and speaker honoraria from Medtronic and Recor. F. Mahfoud is supported by the Ministry of Science and Economy of the Saarland, the Deutsche Hochdruckliga and the Deutsche Gesellschaft für Kardiologie. M. Azizi has received research grants from the French Ministry of Health, Servier, Novartis, and Quantum Genomics, has received honoraria for advisory board meetings from Actelion, and has received speaker’s honoraria from CVRx, Servier, and Astra. A. Pathak has received honoraria for advisory board membership or speaker’s tasks from Ablative Solutions, CVRx, Medtronic, ReCor, Rox Medical and St. Jude Medical. D. Kandzari receives research/grant support and minor consulting honoraria from Medtronic. T. Lüscher has received research grants from Medtronic, Boston Scientific and St. Jude Medical. The other authors have no conflicts of interest to declare.

