
Introduction to the session
This summary report aims to capture the content of the session at EuroPCR 2018 that reviewed the implications for clinical practice of the SPYRAL HTN-OFF MED trial, in order to share a critical analysis of the trial and report the views expressed in the interactive discussion. This article does not constitute an independent review of the topic by the authors.
What was known before SPYRAL HTN-OFF MED?
Hypertension is highly prevalent and is the most important single risk factor for cardiovascular morbidity and death1. Insufficient blood pressure (BP) control to target values and increasing recognition of high rates of covert non-adherence, despite the availability of safe and effective antihypertensive drugs, underlines the need for new non-pharmacological treatment approaches for hypertension2. Moreover, for a challenging subset of patients with multiple drug intolerances and overt non-adherence, device therapy for hypertension could become an important therapeutic option in the future3.
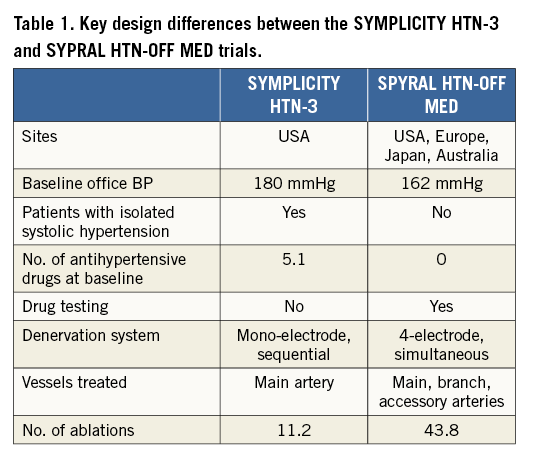
Catheter-based renal sympathetic denervation (RDN) was introduced to interrupt renal sympathetic nerve signalling and lower BP in patients with uncontrolled hypertension4. Several trials and international registries were promising and have demonstrated marked reductions in BP in certain patients5. In contrast, the randomised, sham-controlled SYMPLICITY HTN-3 trial showed no significant difference in BP reduction between patients with resistant hypertension treated with RDN or sham6. This led to a significant setback for the technology. Several aspects of the SYMPLICITY HTN-3 trial including inadequate patient selection, variations in drug adherence and frequent changes of antihypertensive drugs, low operator experience, and inadequate technical performance of the procedure were discussed as potential confounders7. The scientific consensus after the publication of the SYMPLICITY HTN-3 trial and other studies was to recommend that future studies should focus initially on patients with early-stage, combined hypertension5 and that the procedural approach should be revised to include treatment of both main renal arteries and their branches4,8. The key differences between the SPYRAL HTN-OFF MED design and the design of the SYMPLICITY HTN-3 and the SPYRAL HTN-OFF trials are shown in Table 1.
What is SPYRAL HTN-OFF MED about?
The randomised, sham-controlled proof-of-concept SPYRAL HTN OFF-MED trial was designed to address the shortcomings of earlier studies and to provide proof-of-principle for the BP-lowering efficacy of RDN in patients without concomitant antihypertensive drugs9. Briefly, eligible patients were 20 to 80 years old with mild to moderate hypertension (office systolic BP 150-180 mmHg and diastolic BP >90 mmHg, and 24-hr ambulatory systolic BP 140-170 mmHg). Patients were either therapy-naïve or considered to be safely washed-out of their antihypertensive drugs for three to four weeks9. The absence of antihypertensive drug intake as suggested by the protocol was evaluated by serum and plasma toxicological analysis (high-pressure liquid chromatography–tandem mass spectroscopy). Patients were randomised 1:1 to RDN with the Symplicity Spyral™ multielectrode radiofrequency catheter (Medtronic, Minneapolis, MN, USA) (N=38) or sham control (N=42)9. An average of 43.8±13.1 ablations including an average of 25.9±12.8 branch ablations was performed by experienced proceduralists and proctored using detailed treatment plans9. The interim analysis of the first 80 randomised patients showed a significantly greater ambulatory 24-hr systolic BP reduction in favour of RDN at three months (-5.0 mmHg, p=0.0414)9. In the RDN group, office and 24-hr ambulatory BP decreased significantly whereas there were no significant BP changes in the sham-control group (Figure 1)9. Importantly, no relevant adverse events were reported in either treatment group after the three-month follow-up9.
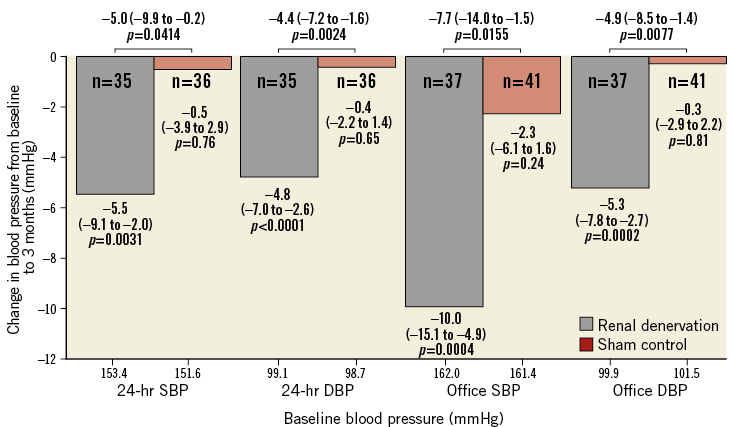
Figure 1. Change in office and ambulatory systolic and diastolic BP for the renal denervation and sham-control groups in the SPYRAL HTN-OFF MED trial at three months. Values are mean (95% confidence interval). P-values are unadjusted. DBP: diastolic blood pressure; SBP: systolic blood pressure. Modified from Townsend et al9, with permission.
Will SPYRAL HTN-OFF MED change my clinical practice?
After a lively discussion, the panellists agreed that SPYRAL HTN-OFF MED, even though not statistically powered for efficacy, provided biological proof-of-principle that radiofrequency-based RDN, as utilised in the study, significantly lowered BP in patients without the confounding effects of antihypertensive medication. Although BP-lowering efficacy was in line with the results of the ultrasound-based RDN system used in the RADIANCE SOLO trial10, which was presented in the Late-Breaking Trial session at EuroPCR 2018, the results of the SPYRAL HTN-OFF MED have to be interpreted in the light of the small number of highly selected participants. Despite focusing on a patient population with a higher likelihood of response and rigorous execution of the study and the procedure, there was still considerable variability in the BP response to RDN, as well as the sham procedure. The impact of distinct patient characteristics, concomitant comorbidities (diabetes, chronic kidney disease, obstructive sleep apnoea), as well as anatomical and procedural factors on outcome remains incompletely understood11. Hence, more patients will need to be treated to improve our understanding of which groups of patients might derive greater or lesser benefit from RDN. Of note, there were significant changes in the procedural algorithm of the SPYRAL HTN-OFF MED trial compared to the SYMPLICITY HTN-3 trial as both main renal arteries and branches with diameters from 3 mm to 8 mm were considered eligible and the total number of ablations increased significantly. Furthermore, until now, no reliable intraprocedural feedback to verify completeness of RDN has been identified; although renal nerve stimulation is under investigation and appears to be of some value12, it may complicate and lengthen the procedure. Furthermore, the durability of the BP-lowering effect needs to be ascertained as in principle, although not yet clinically proven, reinnervation may occur. The investigators emphasised that, in both randomised sham-controlled trials even after deploying a higher number of radiofrequency ablations to the main renal artery and branches, no relevant short-term safety issues were reported5,6,9,10. These safety data are in line with previous reports from real-world registries5.
The alternative to device-based therapy is lifelong oral antihypertensive medication. Unfortunately, non-adherence to antihypertensive medication is a common major issue although antihypertensive drugs are generally well tolerated. Almost one third of all hypertensive patients do not initiate a new prescription of antihypertensive drugs13 and around 50% of them become non-adherent in the first year after initiation of antihypertensive treatment14. A recent survey showed that 8.2% of adults would give up two years of their life rather than add one daily pill, and up to 30% of adults would rather die early than submit to lifelong polypharmacy15. The seriousness and high prevalence of covert non-adherence to antihypertensive medications was underlined by recent device-based therapy of hypertension trials wherein a large proportion of patients was non-adherent despite the awareness of toxicological assessment16,17.
Once confirmed by larger pivotal studies, the encouraging and consistent results from the two recent trials (SPYRAL HTN-OFF MED and RADIANCE SOLO) will provide the first pieces of evidence for the clinical utility of catheter-based RDN in patients unable or unwilling to take antihypertensive medications10,16. Further, the individual patient’s preference concerning whether to take and adhere to lifelong polypharmacy or to undergo an invasive catheter-based procedure with an “always-on” effect has to be taken into account with a view to a more personalised approach to managing hypertension if the BP-lowering effect of RDN is maintained in the long term. The concept of shared decision making is already established in the management of patients with symptomatic atrial fibrillation18.
The Chairperson’s conclusion: where do we stand now?
There remain several areas for further research:
i) Even after eliminating antihypertensive drugs as a confounder, the variability in BP response following RDN has to be understood and reduced.
ii) Renal denervation is still a black box procedure without any direct “read-out” of success.
iii) Identifying which patients may respond best to RDN is critical as this is a high cost procedure regardless of which technology is adopted.
iv) The long-term safety and efficacy of the different technologies has to be assessed.
It will be important to address whether there is additional benefit from disrupting renal nerve signalling other than for BP reduction.
Conflict of interest statement
S. Ewen and F. Mahfoud have received scientific support and speaker honoraria from Medtronic and ReCor. F. Mahfoud is supported by the Ministry of Science and Economy of the Saarland, the Deutsche Hochdruckliga and the Deutsche Gesellschaft für Kardiologie. M. Azizi has received research grants from the French Ministry of Health, Servier, Novartis, and Quantum Genomics, has received honoraria for advisory board meetings from Actelion, and has received speaker’s honoraria from CVRx, Servier, and Astra. P. Lurz has received personal fees from ReCor. M.D. Lobo has received a research grant from Medtronic, speaker honoraria from Cardiosonic, CVRx, Kona Medical and St. Jude Medical, and fees for consulting and advisory board participation from ROX Medical. L. Mauri has received a research grant to the institution and personal fees from ReCor and has received grants to the institution from Abbott, Boston Scientific, and St. Jude Medical, and personal fees from Medtronic. L. Mauri has been employed by Medtronic since June 2018. C. Tsioufis reports honoraria from Pythagoras and Medtronic and personal fees from St. Jude Medical, Medtronic, Elpen, Servier, Boehringer Ingelheim, Novartis, and Win Medica. F. Sharif has received consultancy fees from Medtronic. W. Wijns has received institutional research grants from stent manufacturing companies and speaker fees from Abbott Vascular, Biotronik, MiCell, and MicroPort, is co-founder of Argonauts, an innovation facilitator, and past Board member of Cardio3 BioSciences, now Celyad. L. Lauder has no conflicts of interest to declare.

