Corrigendum:
The legend for Figure 2 has been updated along with an update to the UHF dosage in table 4. - 4th April 2023
Abstract
Since the publication of the 2018 European Society of Cardiology/European Society of Hypertension (ESC/ESH) Guidelines for the Management of Arterial Hypertension, several high-quality studies, including randomised, sham-controlled trials on catheter-based renal denervation (RDN) were published, confirming both the blood pressure (BP)-lowering efficacy and safety of radiofrequency and ultrasound RDN in a broad range of patients with hypertension, including resistant hypertension. A clinical consensus document by the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI) on RDN in the management of hypertension was considered necessary to inform clinical practice. This expert group proposes that RDN is an adjunct treatment option in uncontrolled resistant hypertension, confirmed by ambulatory BP measurements, despite best efforts at lifestyle and pharmacological interventions. RDN may also be used in patients who are unable to tolerate antihypertensive medications in the long term. A shared decision-making process is a key feature and preferably includes a patient who is well informed on the benefits and limitations of the procedure. The decision-making process should take (i) the patient’s global cardiovascular (CV) risk and/or (ii) the presence of hypertension-mediated organ damage or CV complications into account. Multidisciplinary hypertension teams involving hypertension experts and interventionalists evaluate the indication and facilitate the RDN procedure. Interventionalists require expertise in renal interventions and specific training in RDN procedures. Centres performing these procedures require the skills and resources to deal with potential complications. Future research is needed to address open questions and investigate the impact of BP-lowering with RDN on clinical outcomes and potential clinical indications beyond hypertension.
Introduction
High blood pressure (BP) is amongst the most prevalent modifiable cardiovascular (CV) risk factors and remains a leading cause of death1. Despite a stable global prevalence, the absolute number of people with hypertension increased from 648 million in 1990 to 1.28 billion in 20192. Lowering BP through the use of antihypertensive drugs has been shown to reduce the risk for CV morbidity and all-cause mortality34. However, disease awareness and BP control rates remain poor worldwide, especially in low- and middle-income countries and in low-income populations (especially in some ethnicities) residing in high-income countries256.
Over the last two decades, device-based therapies have been investigated as additional treatment options for uncontrolled hypertension. Of these, renal denervation (RDN) has the largest body of evidence for safety and efficacy7. Based on the data available at the time, the 2018 European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) Guidelines for the Management of Arterial Hypertension provided the following recommendation: “Device-based therapies for hypertension are not recommended for the routine treatment of hypertension, unless in the context of clinical studies and randomised controlled trials, until further evidence regarding their safety and efficacy becomes available”8. Since the release of these Guidelines in 20188, several trials have been published providing new evidence (Figure 1)910111213. Hence, a clinical consensus document was deemed necessary by the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). The working group members were equally selected by the ESC Council on Hypertension and the EAPCI. The current paper reviews the evidence for the safety and efficacy of RDN, summarises aspects of the expert group’s discussion, and provides consensus statements for patient selection, centre requirements, procedural aspects, and considerations for future trial designs. In controversial areas, a consensus was achieved by voting and/or agreement of the expert panel after detailed discussions.
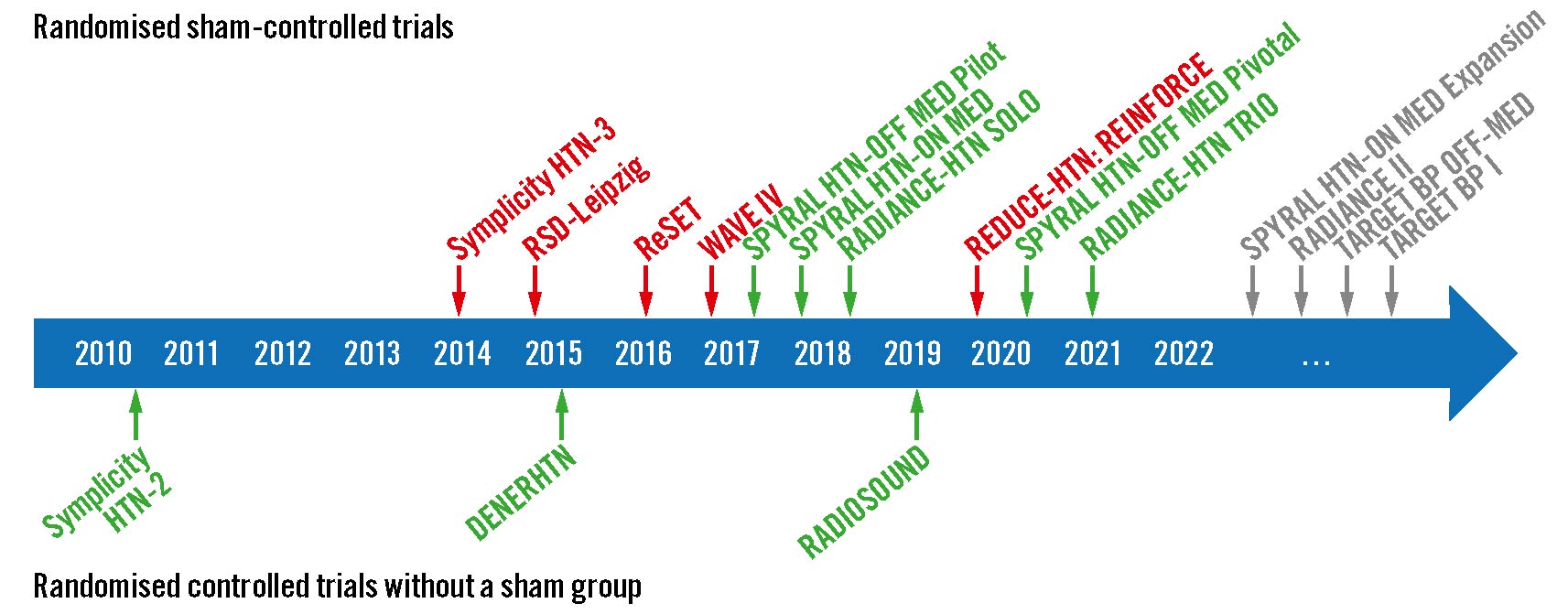
Figure 1. Landmark RDN trials. Overview of important randomised controlled trials with (top) and without (bottom) an invasive sham-control group. Green indicates that the trial met its primary efficacy outcome; red indicates that the trial did not meet its primary efficacy outcome.
Review of clinical data
Table 1 provides the key characteristics of important published randomised clinical trials (RCTs), and Table 2 summarises the characteristics of four ongoing sham-controlled trials investigating RDN for hypertension. These RCTs underwent a rigorous audit evaluating their scientific quality according to the following methodological characteristics: (i) sham-controlled, multicentre trials, (ii) adequate blinding of patients and outcome assessors, (iii) ambulatory BP change as the primary outcome, (iv) study completed as planned with outcome data available for all (or nearly all) randomised participants, and (v) use of second-generation RDN systems and procedural techniques1415.
The highest-quality trials are multicentre, randomised, sham-controlled and blinded (patients and outcome assessors) trials using ambulatory BP as the primary efficacy outcome.
The Symplicity HTN-3 trial did not demonstrate the BP-lowering efficacy of a mono-electrode radiofrequency (RF) catheter system compared with a sham procedure at 6 months16. However, several methodological limitations of this trial, including frequent medication changes, limited training and experience of the proceduralists, likely incomplete circumferential ablation in most patients17, as well as new insights on renal nerve distribution18, informed the design of the second-generation RDN trials. These used revised catheter technologies and procedural techniques in patients with uncontrolled hypertension. Four sham-controlled trials910111213, conducted after the publication of the Symplicity HTN-3 trial, fulfilled all of these methodological criteria (Supplementary Table 1).
In the second generation of sham-controlled trials, RF and ultrasound RDN reduced ambulatory and office BP in patients without (proof of concept) and with antihypertensive drugs (Figure 2)910111213. In three of these RCTs9101113, non-adherence to antihypertensive medications – assessed using ultra-high-performance liquid chromatography-tandem mass spectrometry to detect drugs or their metabolites in blood and urine – was dynamic and frequently observed in both the RDN and the sham groups10. Importantly, RDN lowered BP over the 24-hour circadian cycle, described as an “always-on" effect independent of pharmacokinetics, drug adherence, and dosing schemes (Figure 3). To achieve similar persistent BP-lowering efficacy over 24 hours, antihypertensive medications need to be taken daily and have a long pharmacokinetic/pharmacodynamic half-life. The last published trial conducted in Japan and the Republic of Korea, the REQUIRE trial, did not meet its primary efficacy endpoint of a change in 24-hour ambulatory systolic BP at 3 months due to similar BP reductions in the RDN and sham groups19. When interpreting the trial, several shortcomings in the trial design and conduct have to be considered: i) concomitant antihypertensive medication was not standardised, ii) medication adherence was not objectively assessed, iii) treating physicians were not blinded to treatment allocation, and iv) home and 24-hour ambulatory BP changes were inconsistent19. Importantly, four ongoing sham-controlled RCTs fulfil the above-mentioned scientific quality criteria (Table 2, Supplementary Table 1).
Since the publication of the 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension, several high-quality, randomised, sham-controlled trials910111213 have been published, demonstrating a BP-lowering efficacy over 24 hours for both RF and ultrasound RDN in a broad spectrum of patients whose hypertension ranges from mild-to-moderate to severe and resistant.
Table 1. Key characteristics of important randomised controlled RDN trials.
| Trial, year of publication | Investigational device | Design (randomisation ratio) | Sample size | Inclusion criteria | Primary efficacy outcome | BP reduction in RDN vs control group |
|---|---|---|---|---|---|---|
| Randomised controlled trials | ||||||
| Symplicity HTN-2, 201092 | Symplicity Flex (mono-electrode RF) | Open-label, RDN vs control (1:1) | 106 | Uncontrolled office BP on ≥3 antihypertensive drugs | Change in office SBP at 6 months | −32±23 vs −1±21 mmHg; p<0.0001 |
| DENERHTN, 201593 | Symplicity Flex (mono-electrode RF) | Open-label, SSAHT + RDN vs SSAHT (1:1) | 106 | Uncontrolled office and 24-hr BP on ≥3 antihypertensive drugs | Change in daytime ambulatory SBP at 6 months | −9.9 (95% CI: −13.6 to −6.2) vs −5.9 mmHg (95% CI: −11.3 to −0.5); p=0.033 |
| RADIOSOUND-HTN, 201994 | Symplicity Spyral (multi-electrode RF) vs Paradise (US) | US-RDN vs RF-RDN of the main artery vs RF-RDN of main artery vs RF-RDN of the branches, and accessory arteries (1:1:1) | 120 | Uncontrolled office and 24-hr BP on ≥3 antihypertensive drugs | Change in daytime ambulatory SBP at 3 months | US: −13.2±13.7 mmHg vs RF main artery: 6.5±10.3 mmHg vs RF including branches: −8.3±11.7 mmHg (p=0.043 for US vs RF main artery; p>0.99 for RF main artery vs RF branches) |
| First-generation randomised sham-controlled trials | ||||||
| Symplicity HTN-3, 201416 | Symplicity Flex (mono-electrode RF) | RDN vs sham (2:1) | 535 | Uncontrolled office and 24-hr BP on ≥3 antihypertensive drugs | Change in office SBP at 6 months | −14.1±23.9 vs −11.7±25.9 mmHg; p=0.27 |
| RSD-Leipzig, 201595 | Symplicity Flex (mono-electrode RF) | RDN vs sham (1:1) | 71 | Uncontrolled 24-hr BP on ≥3 antihypertensive drugs | Change in 24-hr SBP at 6 months | −7.0 (95% CI: −10.8 to −3.2) vs−3.5 mmHg (95% CI: −6.7 to −0.2); p=0.15 |
| ReSET, 201696 | Symplicity Flex (mono-electrode RF) | RDN vs sham (1:1) | 69 | Uncontrolled daytimeambulatory BP on ≥3 antihypertensive drugs | Change in daytime ambulatory SBP at 6 months | −6.1 ± 18.9 vs−4.3 ± 15.1 mmHg; p=0.66 |
| WAVE IV, 201797 | Externally deliveredtherapeutic US energy (surround sound system) | RDN vs sham (1:1) | 81 | Uncontrolled office and 24-hr BP on ≥3 antihypertensive drugs | Change in office SBP | −13.2±20 vs−18.9±14 mmHg; p=0.181 |
| REDUCE-HTN: REINFORCE, 202098 | Vessix (multi-electrode RF) | RDN vs sham (2:1) | 51 | Uncontrolled office and 24-hr BP in absence of antihypertensive drugs | Change in 24-hr SBP at 2 months | −5.3 (95% CI: −8.8 to −1.8) vs −8.5 mmHg (95% CI: −13.3 to −3.8); p=0.30 |
| Second-generation randomised sham-controlled trials | ||||||
| SPYRAL HTN-OFF MED Pilot, 20179 | Symplicity Spyral (multi-electrode RF) | RDN vs sham (1:1) | 80 | Uncontrolled office and 24-hr BP in the absence of antihypertensive drugs | Change in 24-hr SBP at 3 months | −5.5 (95% CI: −9.1 to −2.0) vs −0.5 mmHg (95% CI: −3.9 to 2.90); p=0.0414 |
| RADIANCE-HTNSOLO, 201812 | Paradise (US) | RDN vs sham (1:1) | 146 | Uncontrolled daytime ambulatory BPin the absence of antihypertensive drugs | Change in daytime ambulatory SBP at 2 months | −8.5±9.3 vs −2.2±10.0 mmHg; p=0.0001 |
| SPYRAL HTN-ON MED, 201810 | Symplicity Spyral (multi-electrode RF) | RDN vs sham (1:1) | 80 | Uncontrolled office and 24-hr BP on 1 to 3 antihypertensive drugs | Change in 24-hr SBP at 6 months | −9.0 (95% CI: −12.7 to −5.3) vs −1.6 mmHg (95% CI: −5.2 to 2.0); p=0.006 |
| SPYRAL HTN-OFF MED Pivotal, 202011 | Symplicity Spyral (multi-electrode RF) | Bayesian adaptive design, RDN vs sham (1:1) | 331 | Uncontrolled office and 24-hr BP, in the absence of antihypertensive drugs | Change in 24-hr SBP at 3 months | −4.7 (95% CI: −6.4 to −2.9) vs −0.6 mmHg (95% CI: −2.1 to 0.9); p=0.0005 |
| RADIANCE-HTNTRIO, 202113 | Paradise (US) | RDN vs sham (1:1) | 136 | Uncontrolled office and daytimeambulatory BP on 3 antihypertensive drugs | Change in daytime ambulatory SBP at 2 months | −8.0 (IQR −16.4, 0.0) vs −3.0 mmHg (IQR −10.3, 1.8); p=0.022 |
| REQUIRE,202219 | Paradise (US) | RDN vs sham (1:1) | 143 | Uncontrolled office and 24-hr BP on ≥3 antihypertensive drugs | Change in daytime ambulatory SBP at 3 months | −6.6 (95% CI: −10.4 to −2.8) vs −6.5 mmHg (95% CI: −10.3 to −2.7); p=0.971 |
| BP: blood pressure; CI: confidence interval; IQR: interquartile ratio; RDN: renal denervation; RF: radiofrequency; SBP: systolic blood pressure; SSAHT: standardised stepped-care antihypertensive treatment; US: ultrasound | ||||||
Table 2. Ongoing sham-controlled RCTs (as of June 2022).
| Trial, NCT* | Catheter system | Design, (randomisation ratio) | Sample size | Inclusion criteria | Primary efficacy outcome | Estimated trial completion |
|---|---|---|---|---|---|---|
| SPYRAL HTN-ON MED Expansion, NCT02439775 | Symplicity Spyral (multi-electrode RF) | Bayesian adaptive design, RDN vs sham (1:1) | 340 | Uncontrolled office and 24-hour BP on 1-3 antihypertensive drugs | Change in 24-hour SBP at 6 months | 2026 |
| RADIANCE II, NCT03614260 | Paradise (US) | RDN vs sham (1:1) | 225 | Uncontrolled stage II hypertension (office and daytime ambulatory BP) in absence of antihypertensive drugs | Change in daytime ambulatory SBP at 2 months | 2022 |
| TARGET BPOFF-MED,NCT03503773 | Peregrine (ethanol injection via microneedles) | RDN vs sham (1:1) | 90 | Uncontrolled office and 24-hour BP in absence of antihypertensive drugs | Change in 24-hour ambulatory SBP at 2 months | 2023 |
| TARGET BP I,NCT02910414 | Peregrine (ethanol injection via microneedles) | RDN vs sham (1:1) | 300 | Uncontrolled office and 24-hour BP on 2-5 antihypertensive drugs | Change in ambulatory 24-hour SBP at 3 months | 2025 |
| *NCTs found at ClinicalTrials.gov. BP: blood pressure; RDN: renal denervation; RF: radiofrequency; SBP: systolic blood pressure; US: ultrasound | ||||||
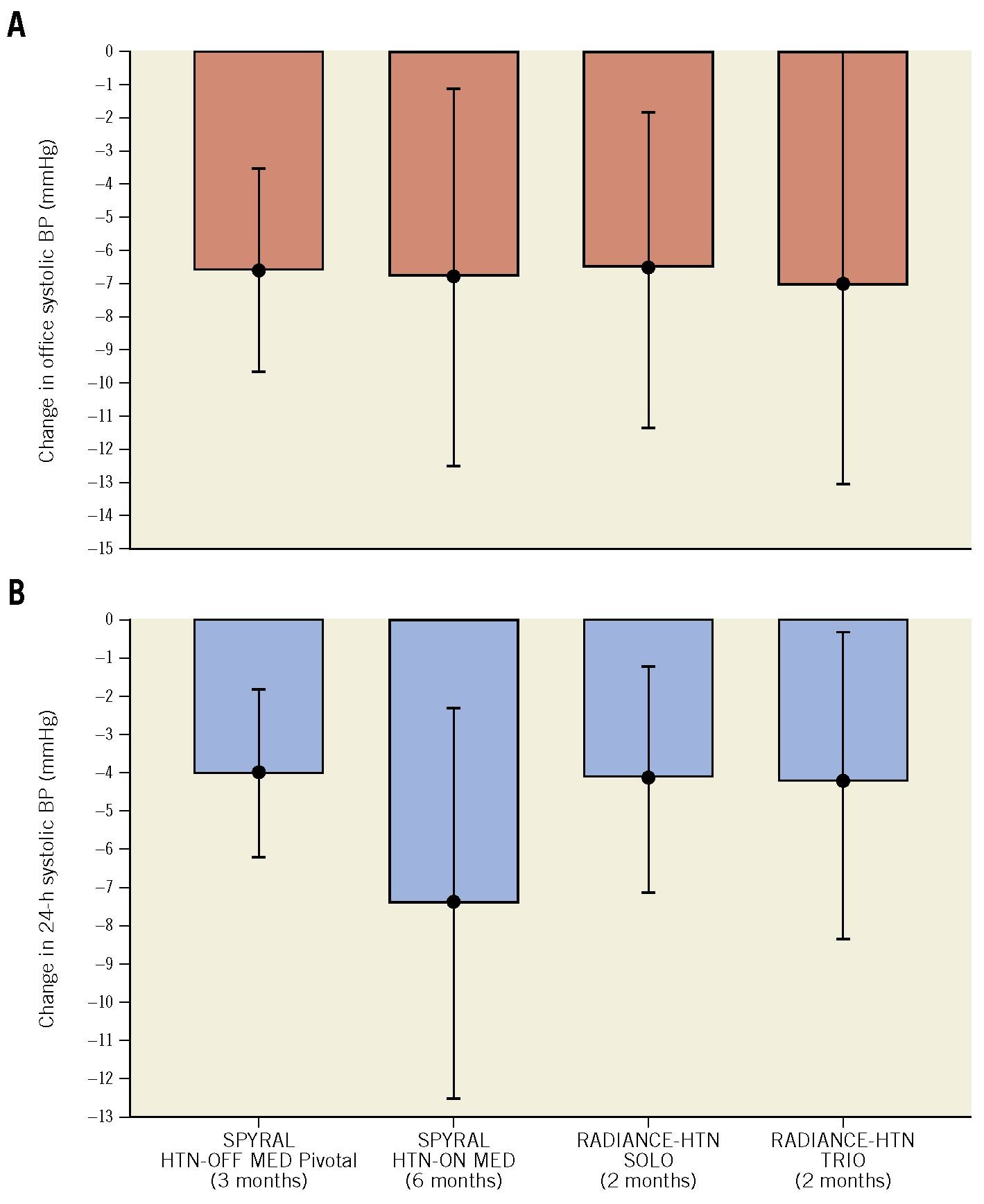
Figure 2. Mean difference in BP change between the RDN and the sham group in second-generation sham-controlled RDN trials.
The mean difference in office (A) and 24-hour (B) systolic BP change between the RDN and the sham group. The SPYRAL HTN-OFF MED Pivotal trial used a Bayesian design with an informative prior (outcome analyses included data from the pilot and pivotal trials). Data are mean and 95% confidence intervals (CI). BP: blood pressure
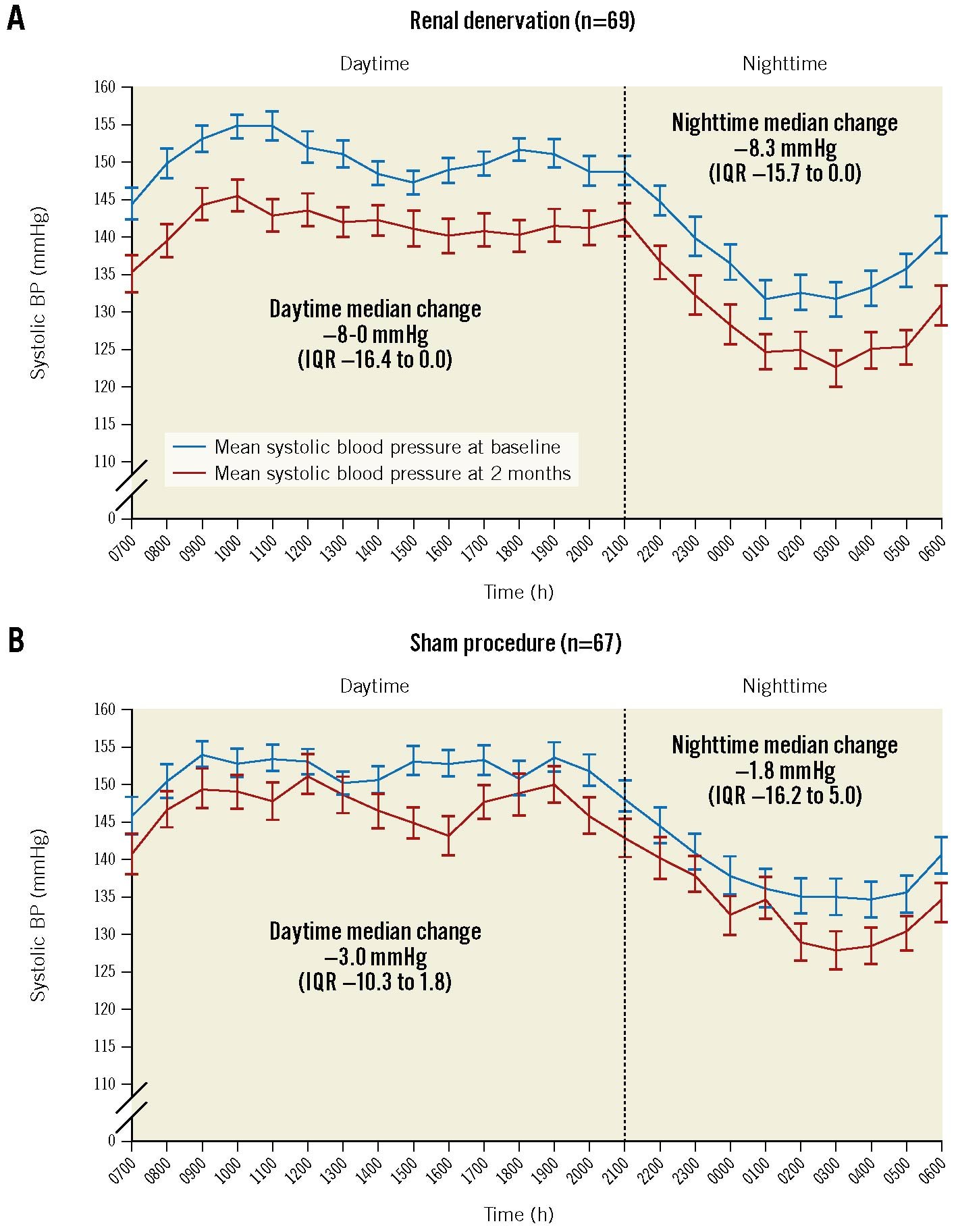
Figure 3. Twenty-four-hour ambulatory BP profile at baseline and 2-month follow-up in the RADIANCE HTN-TRIO trial. Change in systolic BP in the renal denervation (A) and sham groups (B). Hourly BP data are mean±standard errors (SE). Changes between baseline and follow-up are median (interquartile range [IQR]). Adapted with permission from13. BP: blood pressure
SAFETY
In addition to the RCTs, well-conducted registries provide short- and long-term safety data on RDN20. Possible acute procedure-related events are summarised in Table 3. After reviewing the available data (Supplementary Table 2), the experts did not identify any specific safety concerns associated with RDN beyond the expected complication rates of a transfemoral arterial access procedure (less than 1%) and the patients’ exposure to radiation21. In the Symplicity HTN-3 trial, the largest sham-controlled randomised trial investigating RDN, 1 of 364 patients (0.3%) had a vascular access site complication22. The radiation dose varies depending on several factors, including patient characteristics (i.e., obesity, renal artery anatomy), the interventionalist’s experience, and the number of ablation attempts.
There is no evidence of significant procedure-related safety concerns beyond the risks associated with femoral arterial access.
Possible long-term concerns are both the development of de novo renal artery stenosis secondary to vascular injury induced by RDN23 and worsening kidney function. In a meta-analysis of 50 studies including 5,769 patients (10,249 patient-years) undergoing RF-RDN, the pooled annual incidence rate for renal artery stenting was 0.2%24, similar to the reported natural incidence of renal artery stenosis in arterial hypertension25. Importantly, 79% of all events occurred within one year post-procedure24. RCTs systematically using non-invasive renal artery imaging one year after the procedure have been reassuring regarding the vascular safety of RDN91011121316. Moreover, no acute kidney injury or time-dependent decrease in kidney function was reported. A meta-analysis of 48 studies including 2,381 patients showed no significant change in the estimated glomerular filtration rate (eGFR) after a mean follow-up of 9.1 months26. In the Global SYMPLICITY Registry, the observed eGFR decrease over three years was within the expected time-dependent eGFR decline in patients with severe hypertension20. Only 0.3% of the patients without chronic kidney disease at baseline had new onset end-stage kidney disease at the 3-year follow-up27. During the long-term follow-up of the SPYRAL HTN-ON MED trial, changes in eGFR and serum creatinine from baseline to 36 months did not differ between the RDN and sham groups28. In the 4-year follow-up of patients with resistant hypertension included in the Symplicity HTN-3 trial, the rate of new-onset end-stage kidney disease was 5%22. Of note, patients with an eGFR of <40 ml/min/1.73 m2 have been excluded from all sham-controlled trials910111213. Thus, renal safety can only be considered in patients with normal or mildly-to-moderately reduced kidney function (Kidney Disease Improving Global Outcomes [KDIGO] stage G1 to G3a). Another limitation refers to the lack of follow-up extending beyond three years.
Long-term follow-up data up to three years did not reveal any significant increase in de novo renal artery stenosis (<1%) or worsening kidney function beyond the expected rates in hypertensive patients with normal or mildly-to-moderately reduced kidney function.
Table 3. Possible procedural complications and preventive measures.
| Complications | Preventive measures/management strategies |
|---|---|
| Vascular access site complications, e.g., haematoma, pseudoaneurysm, fistula, bleeding, etc. | US-guided puncture, vascular closure device, blood pressure control |
| Contrast-induced acute kidney injury | Adequate (preprocedural) hydration, minimal contrast volume (or diluted contrast) |
| Vascular complications, e.g., renal artery spasm, dissections, distal perforation, intracapsular renal haematoma, renal artery stenosis/dissections, aortic dissection, embolisation | Non-selective abdominal aorta angiogram, no-touch technique to selectively engage the renal artery, avoidance of hydrophilic guidewires, proper RDN technique, intra-arterial injection of a vasodilator, availability of adequately sized stents on site in case of acute renal artery complication which cannot be reversed by prolonged renal artery ballooning |
| RDN: renal denervation; US: ultrasound | |
DURABILITY
There are questions regarding functional reinnervation of the kidneys following RDN. In hypertensive sheep with chronic kidney disease, partial regrowth of renal nerves and return of function were reported 30 months after RDN29. In contrast, permanent axonal destruction and sustained reductions in renal noradrenaline were documented in a porcine model30. Long-term follow-up data from the Global SYMPLICITY Registry20, the SPYRAL HTN-ON MED trial28 and the RADIANCE-HTN SOLO trial31 indicate that the BP-lowering efficacy of RDN in patients with hypertension is sustained for at least up to three years, with a trend for continuous BP reduction over time (Figure 4). The demonstration of durability can be challenging because of dynamic changes in medications, lifestyle interventions, development of coexisting illnesses, ageing, etc.15
Data from registries and sham-controlled trials indicate a sustained BP-lowering effect of RDN for up to three years.
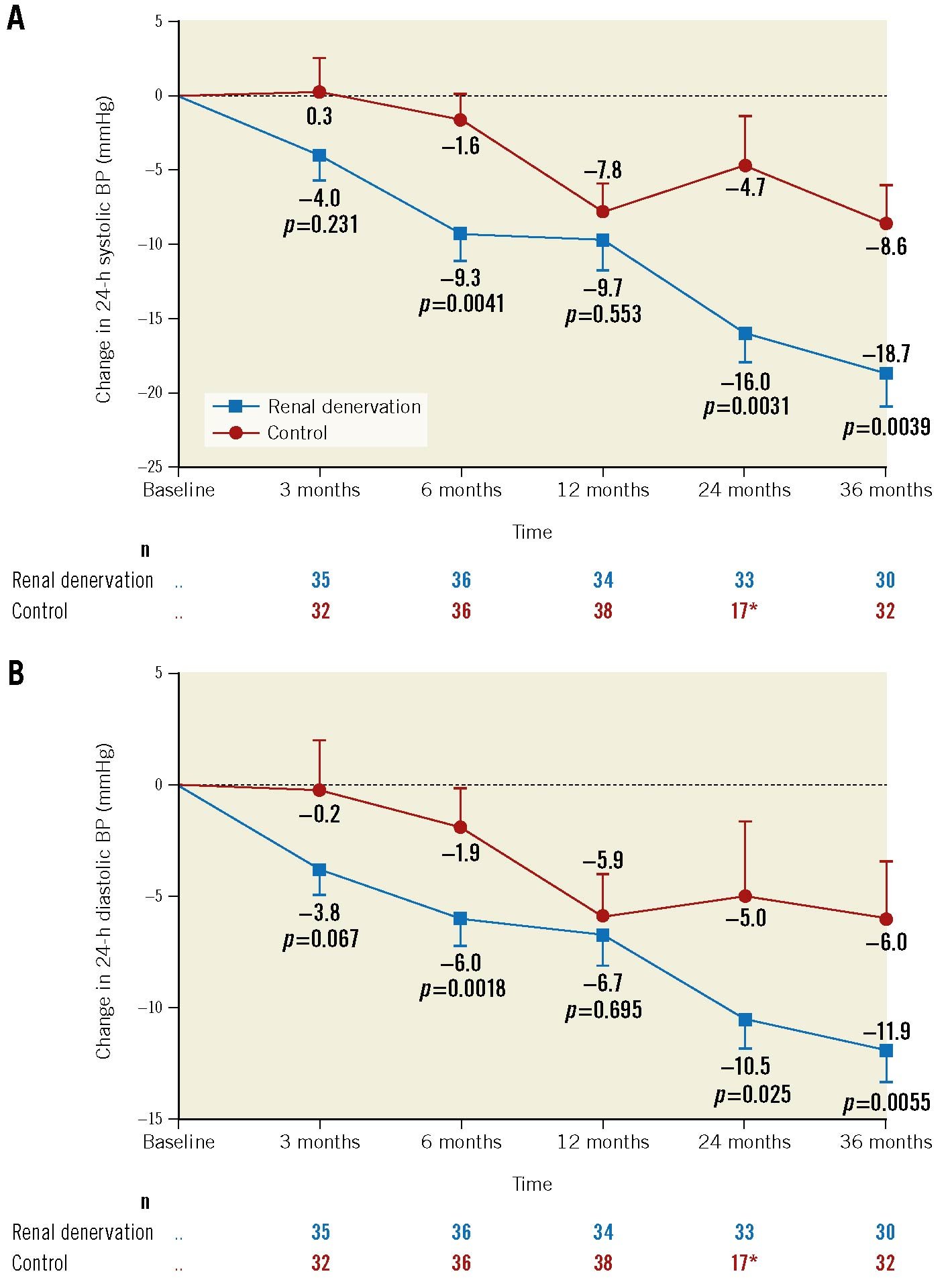
Figure 4. Mean change in 24-hour BP from baseline up to 36 months in the SPYRAL HTN-ON MED trial. A) 24-hour systolic BP. B) 24-hour diastolic BP. *Mean sham-control measurements at 36 months include 13 imputed crossover patients’ BP values from the most recent measurements before the RDN procedure. Error bars are standard errors (SE). Adapted with permission from28. BP: blood pressure
Patient selection
According to the 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension, hypertension is defined as an office systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg measured with a validated oscillometric electronic device using repeated measurements on repeat occasions, confirmed by out-of-office BP measurements, including home BP or ambulatory BP monitoring8. In most patients, BP-lowering treatment is recommended if their office BP exceeds ≥140/≥90 mmHg, taking into account their CV risk, hypertension-mediated organ damage and established CV or renal diseases8. It is recommended to target an office BP of <140/<90 mmHg in all patients, if tolerated. In patients aged <70 years, office systolic BP should be further lowered to 120-129 mmHg, if tolerated832. Lowering systolic BP <130 mmHg in fit older patients might be effective and safe, but BP treatment targets should be individualised for very old and frail patients33. A diastolic BP target of <80 mmHg should be considered for all patients8.
The definition of hypertension and thresholds for treatment initiation (including lifestyle modification and antihypertensive drugs) are based on the 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension8.
Treatment of hypertension traditionally starts with lifestyle modifications, including restriction of sodium intake (<2 g sodium per day), reduction of alcohol (<100 g per week), weight loss, smoking cessation, and regular aerobic exercise8. However, lifestyle modifications should not defer the initiation of antihypertensive medications, especially in patients with grade 3 hypertension and in patients at high or very high CV risk8. In most patients, pharmacotherapy using dual single-pill combination therapy consisting of a renin-angiotensin-system (RAS) blocker (angiotensin-converting enzyme inhibitor or angiotensin receptor blocker) and a calcium channel blocker (CCB) or thiazide/thiazide-like diuretic should be initiated8. Triple-drug combination therapy, including an RAS blocker, CCB, and a thiazide/thiazide-like diuretic, ideally as a single pill, is recommended if BP remains above target8.
Resistant hypertension is defined as uncontrolled office BP (≥140/≥90 mmHg), which is confirmed by out-of-office BP measurements, despite appropriate lifestyle changes and the intake of a triple-drug combination, including a diuretic at maximally tolerated doses8. Diagnosing resistant hypertension requires the exclusion of pseudoresistant hypertension and secondary hypertension causes, including mainly primary hyperaldosteronism, renovascular disease, and chronic kidney disease. A frequently underestimated cause of pseudoresistant hypertension is partial adherence (ranging from 13% to 46%) or full non-adherence (ranging from 2% to 35%) to prescribed antihypertensive therapy834.
Non-adherence to antihypertensive medication represents a major barrier to BP control and should be screened for in all patients with uncontrolled hypertension.
If resistant hypertension is confirmed, low-dose spironolactone (25-50 mg daily) is recommended in addition to the existing triple-drug therapy8. If spironolactone is not tolerated or contraindicated, eplerenone, amiloride, or higher doses of diuretics, beta blockers or doxazosin are recommended8. Of note, eplerenone is not marketed for hypertension in various countries. If eplerenone is used, higher doses (i.e., 50-200 mg daily) may be necessary to achieve a BP-lowering effect35. Many of these fourth-line agents do not have evidence supporting an impact on CV outcomes but have been shown to reduce BP in clinical trials.
RDN IN RESISTANT HYPERTENSION
RDN was shown to reduce BP in adult patients with uncontrolled hypertension in addition to antihypertensive drugs1013, including resistant hypertension13. Supplementary Table 3 summarises the inclusion criteria of completed sham-controlled trials. The available evidence also suggests that RDN has an acceptable safety profile. This is particularly important as the procedural risk of an interventional therapy must not exceed the risk from the underlying condition itself36. According to the available evidence, this expert group suggests considering RDN in patients with uncontrolled hypertension despite treatment with ≥3 antihypertensive drugs in appropriate doses, including a diuretic, confirmed by an out-of-office BP measurement, preferably ambulatory BP measurement, (i.e., resistant hypertension) and an eGFR ≥40 ml/min/1.73 m2. It is strongly advised to exclude secondary causes of hypertension before RDN is considered.
RDN may be used in adult patients with uncontrolled resistant hypertension (office BP ≥140/≥90 mmHg confirmed by 24-hour ambulatory systolic BP ≥130 mmHg or daytime systolic BP ≥135 mmHg) treated with ≥3 antihypertensive drugs and an eGFR ≥40 ml/min/1.73 m2.
Patients who are non-adherent or intolerant to multiple antihypertensive drugs, particularly first-line agents and spironolactone, may also be candidates for RDN. These patients may, therefore, be on fewer than 3 drugs at the time of their selection for RDN due to their prior drug intolerance.
RDN may be a possible treatment option for patients unable to tolerate antihypertensive drugs in the long term or patients who express a preference to undergo RDN in a tailored, shared decision-making process.
Of note, patients with isolated systolic hypertension were excluded from the recent sham-controlled trials910111213. There is evidence from post hoc analyses that patients with isolated systolic hypertension might exhibit a less pronounced BP-lowering effect following RDN3738. However, data derived from the Global SYMPLICITY Registry39 and the RADIOSOUND trial40 demonstrated comparable efficacy in patients with and without isolated systolic hypertension.
In the absence of evidence, it is not advised to perform RDN in kidney transplant recipients or patients with severely impaired kidney function (KDIGO stage G4 and G5), including patients with fibromuscular dysplasia, untreated secondary hypertension, a single functioning kidney or who require haemodialysis.
HYPERTENSION-MEDIATED ORGAN DAMAGE AND CV RISK
The coexistence of other CV risk factors with hypertension4142 exponentially increases the risk of CV events, such as myocardial infarction, stroke, and death4344. Hypertensive patients with coronary artery or cerebrovascular disease have the highest absolute CV risk in whom BP-lowering results in greater absolute risk reductions45. Although high-risk populations (end-stage kidney disease, post-myocardial infarction, heart failure, uncontrolled type II or type I diabetes) were excluded from recent sham-controlled RDN trials, this expert group advises considering the global CV risk, hypertension-mediated organ damage and established CV complications in decision-making since BP control is of the utmost importance in these patients. As recommended by recent guidelines, CV risk may be assessed using the Systematic Coronary Risk Estimation 2 (SCORE2) and Systematic Coronary Risk Estimation 2-Older Persons (SCORE2-OP) risk algorithms for fatal and non-fatal (myocardial infarction, stroke) CV disease32. Moreover, RDN may have beneficial effects beyond the antihypertensive effect in patients with comorbidities associated with increased sympathetic nervous system activation.
The patient’s global CV risk should be evaluated, accounting for hypertension-mediated organ damage and CV complications. High CV risk favours the use of RDN.
PATIENT PREFERENCE
Some patients are unwilling or unable to take antihypertensive drugs or increase their medication burden, especially if they have associated comorbid conditions. Patients recently diagnosed with hypertension and not receiving therapy had the highest preference for RDN46. In a recent survey, 38% of the medication-naïve participants stated that they would prefer RDN over taking antihypertensive drugs47. Even though education and empowerment of patients were shown to have a beneficial effect on drug adherence48, these approaches were often unsuccessful in further reducing BP49. In another survey, patients who regarded hypertension as a major concern strongly preferred RDN46. The amount of BP reduction followed by durability has been identified as the most relevant determinant of patient preference for an antihypertensive therapy (drugs or devices) (Weber M, et al. Patient preferences for interventional and pharmaceutical treatments among US adults with uncontrolled hypertension. TCT 2021. Orlando, FL, USA). Understanding the patients’ situations and exploring their goals and preferences is central to the shared decision-making process. Moreover, the shared decision-making process requires that the patient is well informed about the benefits and limitations of RDN and the possible risks associated with the procedure. The patient should be aware that in all RCTs, a large between-patient variability in BP response to RDN of multiple origins was observed (including lack of post-procedural feedback of effective renal nerve ablation and variability in the procedure, in medications added after RDN, in drug adherence, and in the individual pathophysiology of hypertension). None of the predictors of BP response to RDN reported so far are sensitive and specific enough to allow an individualised patient selection. In light of the available evidence from sham-controlled trials9101112, RDN may be applied in patients with uncontrolled hypertension on fewer than 3 drugs, if they express a strong preference for RDN after intensive counselling on RDN and alternative treatment options, including lifestyle modification and medications.
The decision-making process should incorporate the preference of a well-informed and educated patient. To optimise the shared decision-making, patients must be fully informed about the benefits/limitations and risks associated with RDN.
Centre selection
A multidisciplinary hypertension team (MDHT) should oversee RDN programmes and should include experts on hypertension and percutaneous CV interventions. The MDHT may also involve a clinical cardiologist, angiologist and/or nephrologist in some healthcare systems. The hypertension expert should have a clinical focus on hypertension management and verified expertise in assessing secondary hypertension, ideally recognised as a hypertension specialist by accredited bodies such as the ESH. The interventionalists need specific training in RDN procedures. The MDHT meets regularly and documents the indications of RDN and related management strategies.
Multidisciplinary hypertension teams involving experts on hypertension and percutaneous CV interventions should evaluate the indication and perform RDN.
To qualify for an RDN programme, the centre should have a hypertension outpatient clinic, inpatient ward, radiology division, clinical and hormonal laboratory, catheterisation laboratory, coronary care or intensive care unit, and access to an emergent vascular surgery facility, either onsite or remote.
Training
To set up an RDN centre, extensive training is required, which should include:
- access-site management (i.e., proficiency in femoral artery puncture and haemostasis), radioprotection measures (considering the young age of some patients undergoing RDN), knowledge of digital subtraction angiography, contrast-sparing techniques, renal artery anatomy and nerve distribution (Figure 5), selective renal artery catheterisation, and periprocedural BP management and analgesia/sedation;
- hands-on training using a bench model (demo or simulator) of at least one clinically validated and commercially available device;
- offsite attendance of an active RDN centre to acquire insights on the organisational structure, including the procedure, patient preparation and follow-up;
- performance of at least five proctored RDN cases with each device intended to be used at the site.
The procedure should be performed by a highly skilled interventionalist with experience in renal artery interventions to avoid high complication rates, as observed in renal artery revascularisation trials5051, and to minimise the risk of ineffective treatments related to suboptimal interventions. In some countries, national societies have provided recommendations on the minimum number of renal artery interventions (RDN or angioplasty/stenting) to be performed per site and/or operator48.
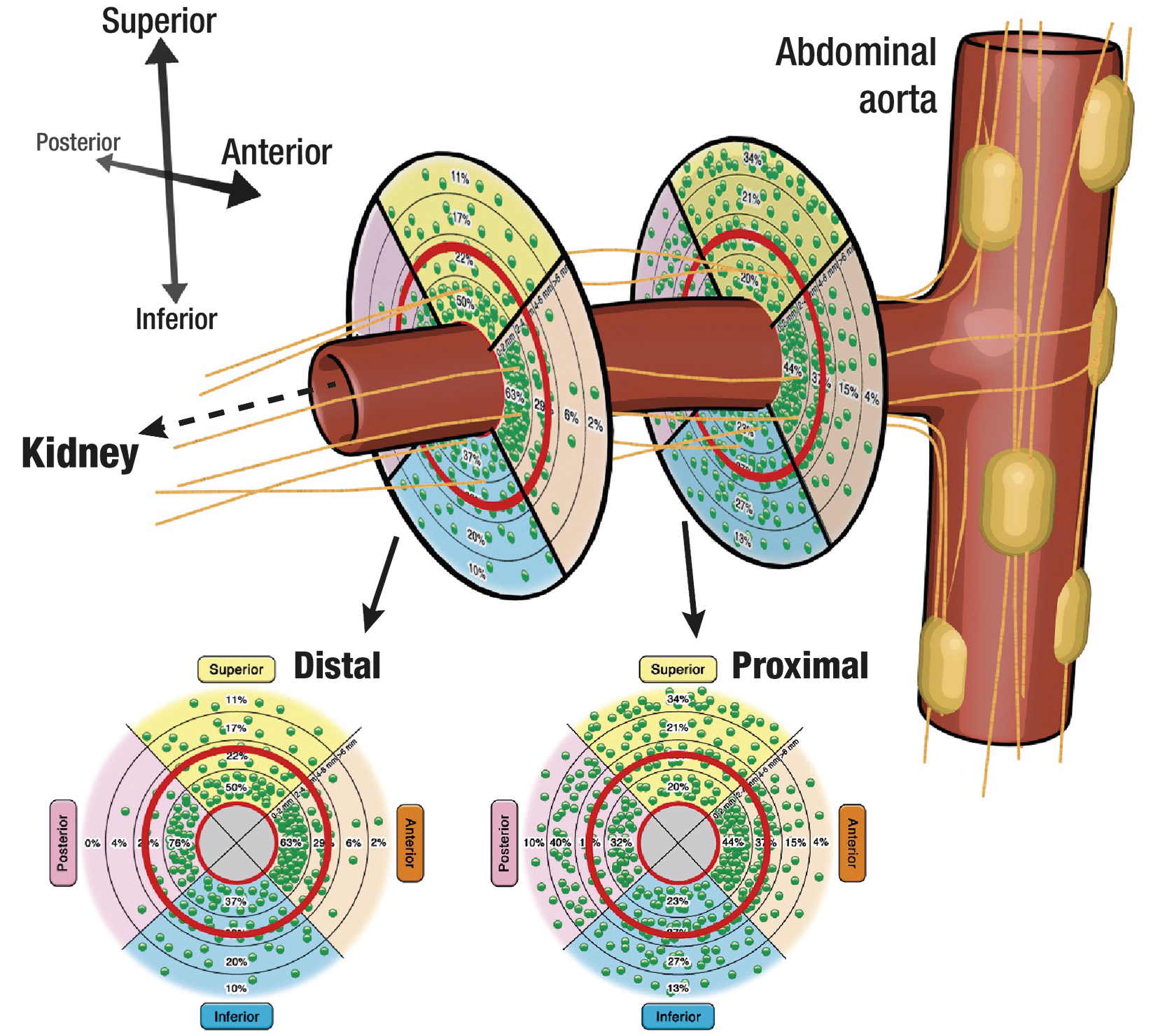
Figure 5. Schematic illustration of the renal artery with its surrounding nerves. The sympathetic nerve fibres originate from the abdominal ganglia and run conically to the distal part of the vessel. The lower circles show the nerve distribution stratified according to the total number (each green dot represents 10 nerves) and relative number (as percent per segment) of nerves. Adapted with permission from91.
Preprocedural imaging
Preprocedural planning should include non-invasive renal artery imaging to anticipate anatomical peculiarities (e.g., presence of accessory arteries) and screen for anatomical ineligibility criteria (e.g., inappropriate vessel diameter), such as untreated severe atherosclerotic renal artery disease or fibromuscular dysplasia. The choice of imaging modality should be based on patient characteristics (e.g., obesity), expected image quality, availability, and local expertise36. Even though duplex ultrasound is preferred as a screening method due to its widespread availability, low costs, and the avoidance of radiation and contrast dye, it is highly observer-dependent and may not provide images of sufficient quality, especially in obese patients. Computed tomography angiography (CTA) or magnetic resonance angiography (MRA) are the preferred imaging procedures that can detect adrenal and renal artery abnormalities, especially in the work-up of patients with resistant hypertension. However, selective renal angiography immediately before RDN remains the gold standard since CTA or MRA may miss some renal artery abnormalities which preclude RDN, such as fibromuscular dysplasia.
Procedural considerations
The required patient preparation is reported in Table 4. Supplementary Table 4 lists the necessary toolbox for RDN procedures. The efficacy and safety of the multi-electrode Symplicity Spyral RDN catheter system (Medtronic) and the Paradise ultrasound catheter system (ReCor) have been documented in sham-controlled trials. The specific features of the devices are outlined in Table 5.
With current-generation RDN devices, femoral arterial access is needed, ideally using sonographic guidance. Successful haemostasis with closure devices is advisable to shorten hospital stays, especially in patients with uncontrolled hypertension who are overweight or obese.
Most RDN procedures can be performed using a single posterior-anterior projection of the kidney. In tortuous anatomies, the ideal placement for energy delivery may be obscured by overlapping vascular branches and, hence, difficult to identify. In these cases, cranial or caudal projections in ipsilateral oblique positions are helpful. Modern angiography systems allow good-quality fluoroscopic image acquisition without cine filming to reduce the radiation dose. A global aortography centred on the kidneys can help identify artery origins and accessory renal arteries. At the end of the RDN procedure, angiography of the renal arteries should be performed to assess potential renal parenchymal or arterial injuries.
Standard operating procedures are suggested for each device to achieve the most effective renal nerve ablation in optimal periprocedural patient security conditions.
Several potential approaches (e.g., transvascular pacing52, arterial flow and resistance53, renal artery vasodilation)54 have been investigated in preclinical and clinical studies to intraprocedurally confirm successful RDN. There is no validated, easily applicable periprocedural clinical indicator of successful renal nerve ablation. Whether this is partly related to the fact that complete interruption of sympathetic nerves surrounding the renal arteries occurs after up to 90 days post-procedure remains to be shown55. Periprocedural complications and possible preventive measures and management strategies are summarised in Table 3.
At present, there is no validated, easily applicable periprocedural clinical indicator of successful renal nerve ablation.
Table 4. Patient preparation.
| Adequate hydration as per contrast media-based procedure |
| Intraprocedural administration UFH (100 U/kg to target ACT >250 sec) |
| Periprocedural administration of an aspirin loading dose, followed by aspirin 75-100 mg for 1 month post-procedure |
| Patients on OAC are managed according to the CCS guidelines related to endovascular interventions99 |
| Analgesia and sedation according to the Monitored Anaesthesia Care approach: low doses of opioids (e.g., morphine 1-3 mg or fentanyl 1-2 mcg/kg intravenously [i.v.]) and benzodiazepine (e.g., midazolam 2-3 mg i.v.) |
| Intraprocedural monitoring of vital parameters |
| Drugs for management of adverse events must be available in the catheter laboratory (e.g., naloxone and flumazenil) |
| Intravenous drugs for blood pressure control (e.g., nitroprusside, urapidil, nitroglycerine, phentolamine) |
| ACT: activated clotting time; CCS: chronic coronary syndrome; OAC: oral anticoagulants; UFH: unfractionated heparin |
Table 5. Specific considerations related to the RDN device.
| Symplicity Spyral RF catheter system | Paradise US catheter system | |
|---|---|---|
| Anatomical eligibility criteria | Treatment of all accessible arteries with a diameter of 3-8 mm | Treatment of accessible main renal arteries with a diameter of 3-8 mm |
| Access | Femoral access (6 Fr) | Femoral access (7 Fr) |
| Wiring | Consider use of extra-support wires or buddy wires in tortuous anatomy | Consider use of extra-support wires or buddy wires in tortuous anatomy |
| Ablation sites | Main renal artery and branches | Main renal artery, 2-3 ablations per artery. The selection of catheter size and ablation site required preprocedural planning with CT/MRA in trials. Final sizing can be done during the renal angiogram before the procedure |
| Arterial wall contact | Ensure appropriate contact of the RF electrodes and the vessel wallEnsure energy delivery (for at least 45 sec, ideally 60 sec) | Ensure complete occlusion of the renal artery after balloon inflation |
| Duration | Simultaneous ablation at 4 points (for at least 45 sec, ideally 60 sec) | 7 seconds per ablation |
| CT: computed tomography; MRA: magnetic resonance angiography; RF: radiofrequency; US: ultrasound | ||
Table 3. Possible procedural complications and preventive measures.
| Complications | Preventive measures/management strategies |
|---|---|
| Vascular access site complications, e.g., haematoma, pseudoaneurysm, fistula, bleeding, etc. | US-guided puncture, vascular closure device, blood pressure control |
| Contrast-induced acute kidney injury | Adequate (preprocedural) hydration, minimal contrast volume (or diluted contrast) |
| Vascular complications, e.g., renal artery spasm, dissections, distal perforation, intracapsular renal haematoma, renal artery stenosis/dissections, aortic dissection, embolisation | Non-selective abdominal aorta angiogram, no-touch technique to selectively engage the renal artery, avoidance of hydrophilic guidewires, proper RDN technique, intra-arterial injection of a vasodilator, availability of adequately sized stents on site in case of acute renal artery complication which cannot be reversed by prolonged renal artery ballooning |
| RDN: renal denervation; US: ultrasound | |
Clinical trial design considerations
Selection of controls: sham or no sham?
The US Food and Drug Administration requires sham-controlled trials for device-based therapies for hypertension, where feasible and ethical56. A recent meta-analysis suggests that the standardised mean difference for primary efficacy outcomes between invasive interventions and sham procedures was small to moderate, which underlines the influence of non-specific effects on trial outcomes and an overestimation of the clinical efficacy of interventions in many circumstances14. Although the risk of adverse events following the sham procedure was low for most trials included in the meta-analysis14, exposing patients to risk by referring them to an invasive sham procedure can raise ethical concerns. The number of patients allocated to a sham procedure should be minimised as much as possible. While some studies suggest that the invasiveness of a sham procedure correlates with its effectiveness57, the necessary invasiveness of a sham procedure in hypertension trials remains unclear. A trial investigating whether each step of the current sham procedure (i.e., skin puncture, femoral/radial access, and angiogram) is needed for the patient’s blinding would be desirable. Importantly, adequate blinding of participants and outcome assessors should be established and assessed58. Further, implementing blinding indices to ensure the absence of bias is advised.
Pooled standardised data from control patients of randomised, sham-controlled trials could be used as an historical control group to avoid exposing patients to invasive placebo procedures and reduce costs59.
For devices approved in certain indications, allocating patients to a sham procedure can be avoided. Comparisons with an active comparator, for example, an already approved device (or drug therapy), could be an alternative.
It is anticipated that future trials comparing two active device treatments could be designed as active-controlled, non-inferiority trials, rather than sham-controlled trials. However, such trials would require larger sample sizes and tight non-inferiority margins for safety and efficacy to be clinically relevant60.
Follow-up duration
A follow-up duration of 8 to 12 weeks was sufficient to demonstrate the BP-lowering efficacy of RDN in the absence of antihypertensive medications91112. However, in contrast to antihypertensive medications, where no further BP decrease is seen after 8 to 12 weeks6162, sustained and meaningful BP reductions were documented up to 36 months after RDN independently from concomitant antihypertensive medication burden2831. Even though renal sympathetic reinnervation is a theoretical concern, regrown nerves do not regain normal function2963. Investigation of longer-term efficacy may be challenging because of i) the unblinding of patients and outcome assessors (performance bias), ii) crossover to RDN of patients initially allocated to the control group, iii) age- and body weight-dependent longitudinal BP changes, iv) the addition of antihypertensive medications to facilitate BP control, v) dynamic changes in drug adherence over time, vi) possible lifestyle modifications, and vii) development of a coexisting illness. A placebo-controlled, randomised withdrawal of antihypertensive medications for a limited period of 4 to 6 weeks could be used to assess long-term efficacy after 12 and 24 months3664. However, assessing BP during a washout period may equally be limited by confounding factors independent of medication adherence.
Well-designed registries with standardised protocols to collect comparable data from one device to another at similar timepoints and follow-up duration and that are regularly monitored for data accuracy and completeness should be conducted to detect adverse events in a real-world setting for up to three years. Registries should allow annual safety, post-market surveillance and performance reports.
Statistical considerations
Adaptive designs modifying the course of a trial following prespecified rules have been introduced in addition to the traditional fixed trial design11. Using an adaptive trial design might be more efficient, resource-saving, and ethically favourable as unnecessary enrolment of patients can be avoided65. While conventional reporting of composite outcomes and time-to-event analyses do not reflect the clinical importance of an event66, hierarchical approaches, such as the Finkelstein-Schoenfeld method67 and the win ratio,66 prioritise more clinically relevant events and allow the combination of BP outcomes with patient-centred or patient-reported outcomes15.
Meta-analyses
This expert group suggests performing an individual patient-level meta-analysis of all second-generation RCTs once the four currently ongoing sham-controlled trials of high scientific quality have been completed. Such a meta-analysis could provide additional information on the preferred target patient groups and facilitate the performance of a robust cost-effectiveness analysis, which might be crucial for implementing RDN in hypertension management across different national healthcare systems. Limitations should be acknowledged, including differing RDN methods, variability in endpoint assessment, and absence/presence of medications, among other factors. An independent academic investigator group should perform such a meta-analysis.
The currently available meta-analyses on RDN aggregate data from studies of different designs and data quality, which may impact the efficacy and safety assessments. The highest-quality meta-analysis requires individual patient-level data from the second-generation RDN trials.
BP outcomes
As BP is a continuous and dynamic variable, office, home, and 24-hour ambulatory BP measurements are complementary approaches to accurately define BP response to treatment68. Office BP measurements are widely available, inexpensive and, if performed according to guidelines8, accurate. Office BP has been used in most landmark hypertension trials and is most commonly used for hypertension management in clinical practice8. Averaging BP determined during several visits might further increase the precision of office BP.
Out-of-office BP, including home and 24-hour ambulatory BP, eliminates the white-coat effect. Home BP predicts CV morbidity and mortality better than office BP69 and might improve medication adherence. Twenty-four-hour ambulatory BP is less prone to bias and regression to the mean. Moreover, 24-hour BP, especially night-time BP, has a stronger association with hypertension-mediated organ damage and CV outcomes than office BP707172. More sophisticated BP measures, including visit-to-visit variability73 and time in the BP target range74, might be useful as additional outcomes of RDN trials. Cuffless wearable devices are currently being validated75 and may be utilised in future trials to assess real-time BP, heart rate, activity and sleeping patterns.
Assessment of medication burden and drug adherence
Knowledge of medication changes and drug adherence is crucial when assessing BP changes following RDN. Non-adherence to antihypertensive medication is common (including approximately 50% of patients with “treatment-resistant hypertension”)34 and associated with poor clinical outcomes76. Assessing drug adherence is complicated by non-uniform usage of definitions and a lack of gold-standard methodology77, reflecting the dynamic changes in adherence over time1049.
While most studies investigating antihypertensive treatment used simplified dichotomous measures to report medication burden (e.g., number of pills, number of medications, number of daily doses), several more detailed indices have been introduced recently to quantify the medication burden (Supplementary Table 5)28. All of these indices have limitations, and none can perfectly reflect the complex pharmacokinetics and dynamic characteristics of interactions between antihypertensive medications28. The use of registry data for dispensed medications over long-term follow-up may provide additional information about adherence and persistence to the prescribed therapy78. Urine and plasma are the most commonly used matrices for assessing drug adherence. Drug adherence monitoring in urine is impacted by the long washout periods of several antihypertensive drugs, which often last longer than multiple half-lives, usually exceeding 24 hours.
Assessment of CV morbidity and mortality
First-line agents recommended by the guidelines have been shown to reduce fatal and non-fatal events. Other antihypertensive treatments (e.g., exercise, metabolic surgery, mineralocorticoid receptor antagonists, clonidine, moxonidine, doxazosin, minoxidil, and hydralazine) are recommended by current guidelines, because these approaches have been shown to lower BP8. Still, their impact on CV outcomes has not been prospectively investigated8. BP-lowering is an accepted surrogate marker of the reduction of CV morbidity and mortality879. In a meta-analysis of individual patient-level data, including data for 344,716 participants from 48 randomised trials of pharmacological BP-lowering medications, a 5 mmHg reduction of systolic BP reduced the risk of major adverse CV events (MACE) by about 10%, irrespective of previous diagnoses of CV disease80. The proportional risk reductions for stroke, ischaemic heart disease, heart failure, and CV death were 13%, 8%, 13%, and 5%, respectively80. There is no suggestion that the clinical benefit achieved through BP-lowering should differ whether achieved by medications, device-based therapies, or their combination. Outcome trials for RDN are challenging to conduct as confounding is likely (changes in adherence, lifestyle modification, etc.), they are expensive, long-term follow-up (>3 years) is required, and the residual risk, as observed in the SPRINT81 and STEP33 trials, is very low nowadays, especially in high-income countries. Of note, we calculated that in order to detect the impact of an intervention that reduces office systolic BP by 10 mmHg, conferring a 20% reduction in MACE3 in an RCT in a population with an annual MACE rate of 3.5%33, would require a randomised sample size of 19,544 patients to achieve a power of 80%, with an overall 2-sided alpha level of 0.05.
Hypertension-mediated organ damage
In the absence of outcome data, conducting well-designed studies and registries investigating the impact of RDN on hypertension-mediated organ damage as an intermediate endpoint, such as left ventricular hypertrophy or urinary albumin excretion, becomes more important. A meta-analysis, including several observational studies, suggested that RDN may improve hypertension-mediated organ damage (regression of left ventricular mass, improved diastolic function)82. However, high-risk patient populations (those with end-stage kidney disease, post-myocardial infarction, heart failure, diabetes mellitus) who might benefit the most from BP-lowering were excluded from most studies.
Patient-related outcomes
In line with the Hypertension Academic Research Consortium (HARC)15, we advocate validating patient-related outcome measures (PROMs) in hypertension and systematically including them in RDN trials using health-related quality of life questionnaires (e.g., the European Quality of Life 5-Dimension 3 Level [EQ-5D-3L] and the short-form health survey [SF36]).
Indications other than hypertension
RDN is under investigation as a complementary approach for indications associated with increased sympathetic nervous system activity beyond hypertension (Figure 6). In patients with paroxysmal atrial fibrillation and uncontrolled hypertension, RDN combined with pulmonary vein isolation (PVI) reduced atrial fibrillation (AF) recurrence compared with PVI alone8384. In several animal models of heart failure, RDN improved autonomic balance, decreased renin-angiotensin system activity, and reduced cardiac remodelling858687. Large prospective trials assessing the safety and efficacy of RDN in disease states other than hypertension are advocated.
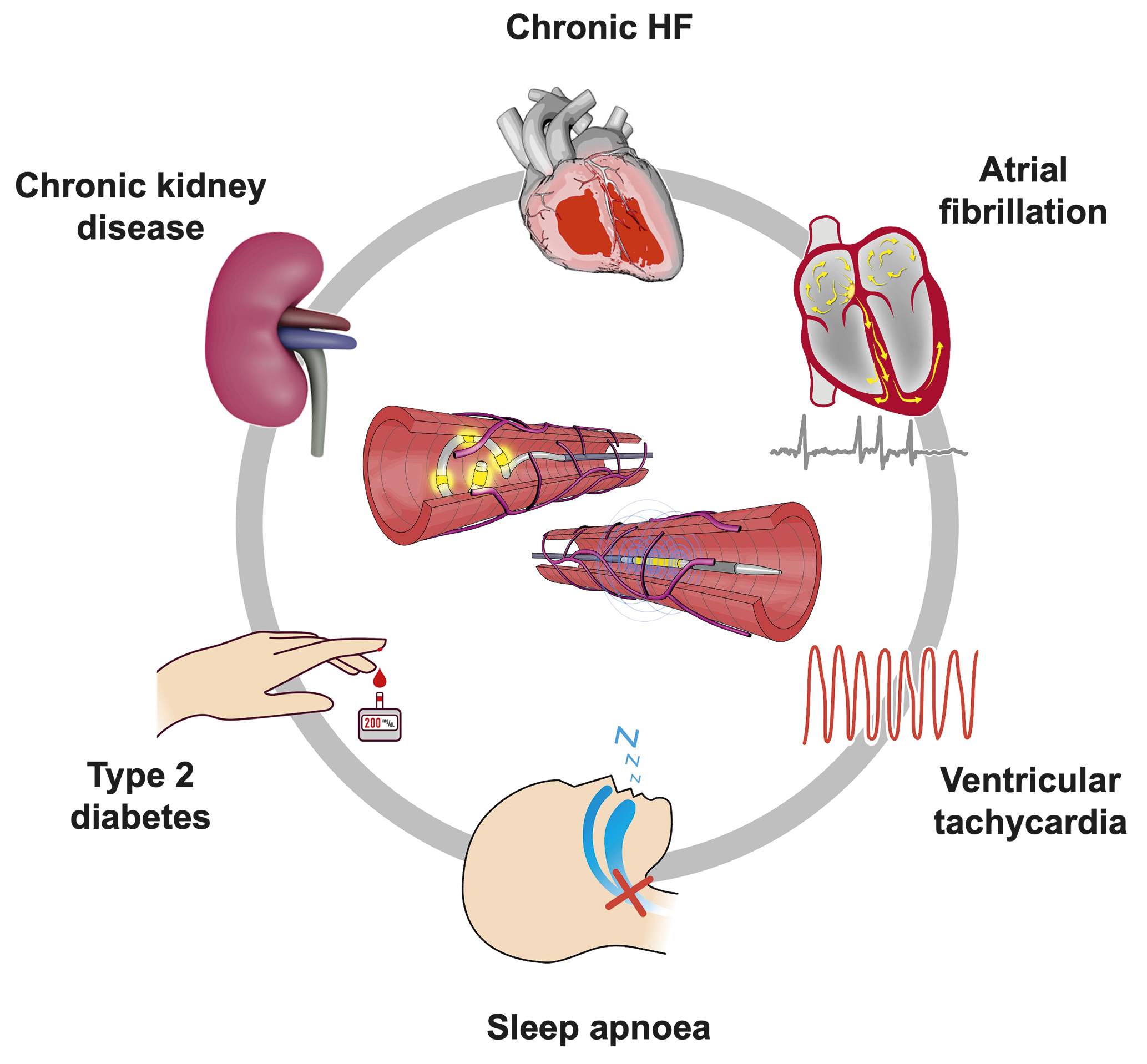
Figure 6. Potential future indications for RDN beyond hypertension (currently under investigation). HF: heart failure; RDN: renal denervation
Impact of COVID-19 on RDN trials
The COVID-19 pandemic has impacted health, lifestyle, and socioeconomic aspects of daily living, which might cause increased variability and variation in BP88 and affect clinical trial conduct by various means (Supplementary Figure 1)888990. Surveys incorporating the patient’s self-reported health status and depression may provide additional perspective on observed BP patterns.
Open questions
Although sham-controlled trials have confirmed the BP-lowering efficacy and safety of RDN in patients without and with antihypertensive drugs, including patients with treatment-resistant hypertension, several questions remain unanswered. First, other than high baseline BP, none of the investigated patient characteristics, haemodynamic parameters or biomarkers have been identified as a consistent predictor for treatment response. Second, there is no simple and reliable method to confirm successful RDN intraprocedurally. Third, the usefulness of repeat RDN among individuals with persistent uncontrolled hypertension has not been investigated. Fourth, while radial arterial access has been established for percutaneous coronary intervention and subsequently demonstrated to lead to a lower risk of access-site complications, no dedicated catheter system is yet commercially available for transradial RDN. Fifth, the value of sympathetic denervation of organs besides the kidney is unclear and remains to be investigated. Sixth, well-designed cost-effectiveness studies for RDN are lacking.
Conclusions
Since the publication of the 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension, several sham-controlled trials of high methodological quality have been published, demonstrating the safety and the BP-lowering efficacy of RF and ultrasound RDN. Therefore, RDN now represents another treatment option in adult patients with uncontrolled resistant hypertension confirmed by ambulatory BP measurements. RDN may also be used in selected patients deemed intolerant to antihypertensive drugs long term following an expert review. The shared decision-making process should incorporate the preference of a well-informed patient and individual CV risk. MDHTs involving experienced experts on hypertension and percutaneous CV interventions should evaluate the indication and perform RDN. Proceduralists require expertise in renal interventions and specific training in RDN procedures, and centres performing RDN should be able to treat any potential complications. Ongoing studies and future research might answer open questions and are needed to investigate RDN for indications other than hypertension.
Acknowledgements
The Coordination Committee consists of Michel Azizi, Emanuele Barbato, Lucas Lauder, Felix Mahfoud, Roland E. Schmieder, Costas Tsioufis, and William Wijns. We are grateful to Armin Schweitzer for his help with the artwork. We would also like to thank Claire Jackson-Blanchet from the Europa Group for her assistance with organisation and logistics.
Conflict of interest statement
E. Barbato has received speaker honoraria from BSCI, Abbott, OpSens, and Insight Lifetech. M. Azizi has received grant support and non-financial support from ReCor Medical and Idorsia; and has received consulting fees from Medtronic, AstraZeneca, Alnylam Pharmaceutical, Poxel Pharma, and Novartis. R.E. Schmieder has received scientific support and grants from Medtronic, ReCor Medical, and Ablative Solutions to the institution; has received honoraria for lectures from Ablative Solutions, Apontis, AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, MENARINI, Medtronic, Novo Nordisk, Novartis, ReCor Medical, and Servier; and honoraria for advisory board activities from Ablative Solutions, Apontis, AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, MENARINI, Medtronic, Novo Nordisk, Novartis, ReCor Medical, and Servier. L. Lauder reports speaker honoraria from Medtronic and ReCor Medical. M. Böhm reports personal fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Vifor, Servier, Medtronic, and Novartis; and grants from Deutsche Forschungsgemeinschaft and AstraZeneca. S. Brouwers has received speaker honoraria from Sanofi, Daiichi Sankyo, Servier, MENARINI, and Merck through the institution. R.M. Bruno is supported by H2020 InSiDe (grant agreement No 871547) and has received speaker honoraria from Medtronic. T. Kahan reports research grants to the Karolinska Institute from Medtronic and ReCor Medical, all outside the submitted work. D.E. Kandzari has received institutional research and grant support from Medtronic and Ablative solutions; personal consulting honoraria from Medtronic and Ablative Solutions; and has equity in BioStar Ventures, but none related to Ablative Solutions. T.F. Lüscher has recieved research and educational grants to the institution from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Novartis, Sanofi, Servier and Vifor, outside this work; and honoraria from Abbott India, Acthera, Cor2ED, Dacadoo, Daiichi Sankyo, Novo Nordisk and Pfizer, unrelated to this work. G. Parati reports speaker honoraria from Omron Healthcare, Bayer, and Servier. A. Pathak reports consultancy/speaker honoraria from Medtronic, ReCor Medical, and Ablative Solutions. F.L. Ribichini has received scientific support and speaker honoraria from Medtronic. M.P. Schlaich is supported by an NHMRC Research Fellowship and has received consulting fees, and/or travel and research support from Medtronic, Abbott, ReCor Medical, Novartis, Servier, Pfizer, and Boehringer Ingelheim. A.S.P. Sharp reports consultancy/speaker honoraria from Medtronic, ReCor Medical, Philips, and Boston Scientific. I. Sudano has received consulting fees from Amgen, AstraZeneca, Daiichi Sankyo, MSD, Medtronic, Novartis, Novo Nordisk, Recordati, Sanofi, and Servier; travel grants from Amgen, AstraZeneca, Daiichi Sankyo, MSD, Medtronic, Novartis, Novo Nordisk, Recordati, Sanofi, and Servier; and honoraria from Amgen, AstraZeneca, Daiichi Sankyo, MSD, Medtronic, Novartis, Novo Nordisk, Recordati, Sanofi, and Servier. M. Volpe has received honoraria from the speakers bureaus at AstraZeneca, Novartis, Boehringer Ingelheim, Bayer, and Amgen; has received payments for scientific collaborations from MENARINI, Servier, Novo Nordisk, Novartis, and Sanofi; has a contract of collaboration with Medtronic but did not receive fees; is a member of the Kalos Medical advisory board. C. Tsioufis has received grants or honoraria from Medtronic, ReCor Medical, AstraZeneca, Bayer, Boehringer Ingelheim, MENARINI, Elpen, Win Medica, Vianex, Novartis, and Servier. W. Wijns reports institutional grants and honoraria from MicroPort; and is the co-founder of Argonauts, an innovation facilitator. F. Mahfoud is supported by Deutsche Gesellschaft für Kardiologie (DGK), Deutsche Forschungsgemeinschaft (SFB TRR219), and Deutsche Herzstiftung. He has received scientific support (to the institution) from Ablative Solutions, Medtronic, and ReCor Medical; and speaker honoraria from Ablative Solutions, AstraZeneca, Bayer, Boehringer Ingelheim, Inari, Medtronic, Merck, and ReCor Medical. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

