Abstract
Arterial hypertension is a global leading cause of cardiovascular, cerebrovascular, and renal disease, as well as mortality. Although pharmacotherapy is safe and effective in lowering blood pressure (BP) and cardiovascular disease risk, BP control remains poor, and the mortality rates associated with high BP have been steadily increasing. Device-based therapies have been investigated to overcome barriers to pharmacotherapy, including non-adherence and low rates of persistence to daily medications. Among these device-based therapies, catheter-based renal denervation (RDN) has been most extensively examined over the past 15 years. In this state-of-the-art article, we summarise the rationale for RDN, review the available evidence, provide recommendations for a safe procedure, and discuss the role of RDN in current guidelines and clinical practice.
Several device-based therapies have been developed for the treatment of hypertension1. Of these, the largest amount of evidence exists for catheter-based renal denervation (RDN)2. The antihypertensive effect of RDN results from modulating the sympathetic nervous system by interrupting afferent and efferent sympathetic renal nerves in the adventitia and perivascular fat of the renal arteries2. More than a decade ago, excitement was created around RDN due to very pronounced blood pressure (BP) reductions in patients with severe, apparent treatment-resistant hypertension in early open-label registries and randomised controlled trials12. This excitement ended abruptly after the first sham-controlled trial − Symplicity HTN-3 − demonstrated the procedure’s safety but failed to show superiority in reducing BP using a monoelectrode radiofrequency (RF) catheter compared with a sham procedure (selective renal angiography only)3. In retrospect, the first-generation sham-controlled trials, which have been extensively discussed previously1, have provided important information on trial design, execution, and conduct. The detailed methodological review of these trials45, new insights on renal nerve distribution67, the refinement of existing catheter systems and the development of new ones paved the way for the second generation of sham-controlled trials. The European Society of Cardiology’s (ESC) Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI) clinical consensus statement on RDN in the management of hypertension judged trials to be of high quality if all of the following methodological characteristics were fulfilled: (i) sham-controlled, multicentre design; (ii) adequate blinding of patients and outcome assessors; (iii) ambulatory BP change as the primary outcome; (iv) study completed as planned with outcome data available for all (or nearly all) randomised participants; and (v) use of second-Âgeneration RDN systems and procedural techniques2.
This review aims to discuss the rationale for RDN and to summarise the outcomes of recent second-generation sham-controlled trials.
Rationale
The autonomic nervous system regulates cardiac output and BP to maintain organ perfusion. Increased sympathetic nervous system activity increases BP through several mechanisms, including peripheral vasoconstriction, venous capacitance reduction, and reduction of renal sodium and water excretion8. Sympathetic activation is generated by the nucleus tractus solitarius and rostral ventrolateral medulla, affecting all peripheral organs9. The kidneys play a central role in BP regulation. The sympathetic nerve fibres originate from the abdominal ganglia and accompany the renal arteries while converging to the arteries’ adventitia from proximal to distal67. The activation of efferent sympathetic renal nerve fibres causes renin release via beta-1 adrenergic receptor activation at the level of the juxtaglomerular cells, increases renal tubular sodium reabsorption via alpha-adrenoceptors, and decreases renal blood flow10. Renal afferent sympathetic nerves respond to renal injury via parenchymal nociceptive receptors11 and changes in pelvic pressure via pressure-sensitive receptors10.
In the first half of the 20th century, surgical sympathectomy was used to treat severe hypertension as an alternative to antihypertensive medications, which at that time had limited availability and were poorly tolerated. Surgical sympathectomy reduced BP and was associated with improved survival in patients with severe hypertension12 at the cost of severe side effects, including postural and postprandial hypotension and syncope, incontinence, and sexual dysfunction, as well as high perioperative morbidity and mortality (ranging from 0.7% to 10.9%)13. However, surgical sympathectomy proved the important role of the sympathetic nervous system for BP regulation.
Radiofrequency renal denervation
Most RDN systems use RF energy applied via mono-3 or multiÂelectrode141516 catheters to thermally ablate renal sympathetic nerves (Table 1). Of these systems, the most data are available for the multielectrode Symplicity Spyral catheter (Medtronic).
Table 1. Characteristics of the most important RDN catheter systems.
| Catheter | Design | Access site | Ablation sites | Efficacy confirmed in sham-controlled trial? |
|---|---|---|---|---|
| Radiofrequency | ||||
| Symplicity Spyral (Medtronic) | Multielectrode (4 monopolar gold electrodes), helical design, rapid exchange monorail catheter, 60 seconds per ablation cycle | F (6 Fr) | Main and accessory arteries, including branches(diameter 3-8 mm) | Yes, multiple trials |
| Netrod (Shanghai Golden Leaf Medtec) | Multielectrode (6 electrodes), basket-shaped tip,120 seconds per ablation cycle | F (8 Fr) | Main and accessory arteries, including branches(diameter 3-12 mm) | Yes, single study (EuroPCR 2023, publication pending) |
| Iberis 2nd-generation (AngioCare and Terumo) | Multielectrode (4 monopolar electrodes), helical design, over-the-wire catheter, 60 seconds per ablation cycle, 90 cm catheter length for transfemoral and 160 cm for transradial RDN | F/R (6 Fr) | Main and accessory arteries, including branches(diameter 3-8 mm) | Yes, single study (CIT Congress 2023, publication pending) |
| SyMapCath I | Steerable monoelectrode stimulation and ablation catheter, stimulation time 20-120 seconds, 120 seconds per ablation cycle | F (6-7 Fr) | Main renal arteries | Yes, single study |
| Ultrasound | ||||
| TIVUS (SoniVie) | Unidirectional steerable or multidirectional, over-the-wire, 30 seconds per emission | F (6 Fr) | Main and accessory arteries (diameter ≥4 mm) | No |
| Paradise (ReCor Medical) | Piezoelectric ceramic transducer within a fluid-cooled, low-pressure balloon, over-the-wire, 7 seconds per emission | F (7 Fr) | Main and accessory arteries (different catheter sizes for diameters of 3-8 mm) | Yes, multiple studies |
| Neurolysis | ||||
| Peregrine (Ablative Solutions) | 3 extendable microneedles | F (7 Fr) | Main and accessory arteries (4-7 mm) | No, TARGET-BP I ongoing |
| F: femoral; Fr: French; R: radial; RDN: renal denervation | ||||
SYMPLICITY SPYRAL RADIOFREQUENCY CATHETER SYSTEM
The SPYRAL HTN clinical trial programme was initiated with two international, multicentre, sham-controlled pilot trials (not powered for efficacy outcomes) investigating the Symplicity Spyral catheter system; each trial included 80 patients with mild-to-moderate hypertension, one trial with (SPYRAL HTN-ON MED) and one without (SPYRAL HTN-OFF MED) concomitant antihypertensive pharmacoÂtherapy1718. Both trials reported significant BP-lowering effects following RDN and only minor BP changes in the sham treatment group1718.
Subsequently, two international randomised, sham-Âcontrolled trials − prospectively powered to detect a change in 24-hour systolic BP − were conducted in patients with mild-to-moderate hypertension in the presence (SPYRAL HTN-ON MED Expansion) or the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal)19. These trials used an adaptive Bayesian design to each include 80 patients from the pilot trials as an informative prior and used supporting interim analyses that allowed early stopping for efficacy or futility19. The SPYRAL HTN-OFF MED Pivotal trial demonstrated the superiority of RF-RDN compared with sham for reducing BP in the absence of concomitant antihypertensive medications16. The primary and secondary effectiveness outcomes were met. Compared with sham, RDN lowered 24-hour and office systolic BP after 3 months by 3.9 mmHg (95% Bayesian credible interval [BCI]: 1.6 to 6.2) and 6.5 mmHg (95% BCI: 3.5 to 9.6), respectively, with a posterior probability of superiority>0.999 for both outcomes (Figure 1)16.
In contrast to the other trials from the SPYRAL HTN trial program, the SPYRAL HTN-ON MED Expansion trial did not show a significant treatment difference for 24-hour systolic BP between the RDN and sham control groups at 6 months (primary efficacy outcome −0.03 mmHg, 95% BCI: −2.82 to 2.77; posterior probability of superiority: 0.51)20. While the 24-hour BP reductions in the RDN groups were consistent across the trials, the BP reduction in the sham group was unexpectedly large (Figure 1B). Prespecified analyses identified a disproportional intensification of medication in the sham group up to 6 months, particularly in patients treated in the USA2021. Moreover, 24-hour BP patterns differed substantially between patients enrolled before and during the COVID-19 pandemic20. Ultimately, due to substantial differences between the pilot and expansion groups, nearly all the pilot data for both the sham and treatment groups were discounted as informative prior for the primary analysis. Of note, several secondary efficacy outcomes were met, including reductions in office systolic (adjusted treatment difference −4.9 mmHg, 95% confidence interval [CI]: −7.91 to −1.89; p=0.002) and diastolic BP (−2.0 mmHg, 95% CI: −3.9 to −0.1; p=0.04), which were greater in the RDN group than in the sham group (Figure 1A)20. When analysing the hourly changes in ambulatory systolic and diastolic BP, BP reductions during the night were larger in the RDN than in the sham group, while daytime BP reductions were similar20. These reductions in night-time BP are particularly important since night-time BP is closely associated with adverse cardiovascular events and hypertension-mediated organ damage22.
Long-term follow-up data from the Global SYMPLICITY Registry23, predominantly using the monoelectrode Symplicity Flex catheter system (Medtronic), suggest BP-lowering effects for up to three years. The SPYRAL HTN-ON MED trial demonstrated a similar BP-lowering effect up to three years of follow-up24, and several single-centre, open-label studies reported sustained BP reductions for up to ten years2526.
Both the first- and second-generation studies of RF-RDN have proven the safety of the procedure, and concerns about deteriorating kidney function and the occurrence of renal artery stenosis following RDN could be dispelled. The available studies do not report acute kidney injury or relevant time-Âdependent decreases in kidney function2327. Patients in sham-controlled RF RDN trials had normal or mild-to-moderately reduced kidney function at baseline (with an estimated glomerular filtration rate [eGFR] >45 ml/min/1.73 m2)2. A meta-analysis including studies published until January 2019, with data from 5,769 subjects with 10,249 patient-years of follow-up, reported a pooled annual incidence rate for stent implantation following RF-RDN of 0.2%28, which is comparable with the reported natural incidence of renal artery stenosis in hypertension29. The SPYRAL HTN-OFF MED Pivotal16 and SPYRAL HTN-ON MED Expansion trials, which were not included in the meta-analysis, also confirmed the safety of the method. The rate of major adverse events at one month among the first 253 patients treated with RDN in the SPYRAL HTN-OFF and -ON MED trials was 0.4% (1/253)20. Importantly, no adverse device-related events were observed. One patient randomised to RDN in the SPYRAL HTN-ON MED Expansion Study underwent a femoral pseudoaneurysm repair at the access site20. Altogether, the procedural risk of RF-RDN is similar to the expected complication rate of other elective transfemoral arterial access procedures (1-2%)28. The SPYRAL AFFIRM trial is currently evaluating the long-term safety and efficacy of the Symplicity Spyral catheter system in up to 1,000 real-world patients with uncontrolled hypertension and is powered for subgroups, including diabetes type 2, isolated systolic hypertension, and chronic kidney disease (ClinicalTrials.gov: NCT05198674). The U.S. Food and Drug Administration (FDA) approved the Symplicity Spyral RDN system for treating hypertension based on the available evidence.
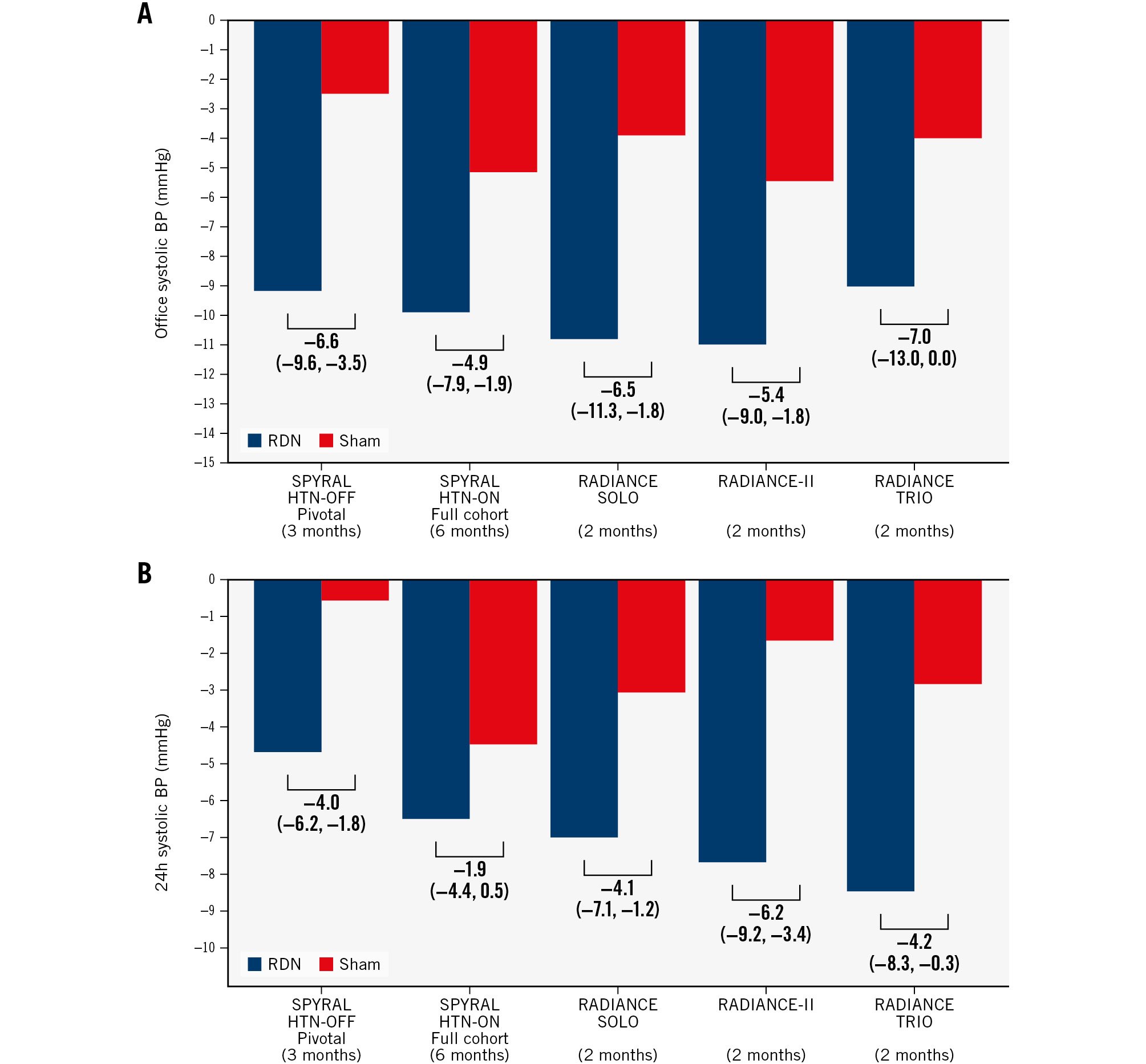
Figure 1. BP changes in second-generation sham-controlled RDN trials. Mean change (95% confidence intervals) in office (A) and 24-hour (B) systolic BP in published second-generation sham-controlled RDN trials. The SPYRAL HTN-OFF Pivotal and SPYRAL HTN-ON trials used a Bayesian design with an informative prior (outcome analyses included data from the pilot and pivotal trials). BP: blood pressure; RDN: renal denervation
NETROD CATHETER SYSTEM
The Netrod catheter system (Shanghai Golden Leaf MedTec Co., Ltd) consists of six electrodes mounted in a spiral array on individual wires. The basket-like tip is adjustable to ensure wall contact in 3-12 mm diameter vessels (Table 1).
A Chinese, single-centre, open-label, first-in-human study, in which 15 patients (93% male) who had uncontrolled hypertension (systolic 24-hour ambulatory BP 145-170 mmHg) after discontinuation of antihypertensive drugs and who subsequently underwent RDN, indicated the feasibility and safety of the procedure, as no serious adverse events occurred during follow-up30.
The results of a prospective, multicentre, randomised sham-controlled trial investigating the safety and efficacy of the Netrod catheter system in China in patients aged 18-65 years with uncontrolled hypertension (office BP 150-179/90-109 mmHg and mean 24-hour BP ≥135 mmHg) and who had received a standardised two-drug antihypertensive treatment of nifedipine and hydrochlorothiazide were presented at EuroPCR 2023 (ClinicalTrials.gov: NCT03261375). Between baseline and six months, office systolic BP (between-group difference: −19.0 mmHg, 95% CI: −23.0 to −15.0; p<0.001) and mean 24-hour systolic BP (−8.7 mmHg, 95% CI: −12.4 to −5.0; p<0.001) were significantly lower in the RDN group (n=139) compared with the sham group (n=66). Compared with previous sham-controlled trials1831, the patients were slightly younger (mean age 50 years; patients >65 years were excluded) and had a higher baseline heart rate (mean of 78 bpm). Both characteristics might reflect high activity of the sympathetic nervous system. Importantly, the procedure was safe, and no renal artery stenosis occurred within six months post-procedure.
IBERIS-HTN (2ND GENERATION)
The Iberis 2nd-generation RF catheter system (AngioCare and Terumo) consists of an over-the-wire catheter with four electrodes arranged in a spiral configuration (diameter 10.5 mm). The catheter system is the first with European conformity (CE)-marking for transradial RDN and comes in two lengths: 160 cm for radial and 90 cm for femoral access.
The Renal Denervation by Iberis MultiElectrode Renal Denervation System in Patients With Primary Hypertension (Iberis-HTN) trial, a randomised, patient and outcome assessor-Âblinded, sham-controlled trial, investigated the safety and efficacy of transfemoral RDN using the Iberis 2nd generation catheter system in 16 Chinese centres. The trial included patients (18-65 years) who were on a standardised triple-drug antihypertensive therapy consisting of amlodipine 5 mg and a two-drug fixed-dose single-pill combination of valsartan 80 mg and hydrochlorothiazide 12.5 mg and who had a systolic BP of 150-180/≥90 mmHg and a mean ambulatory 24-hour systolic BP ≥135 mmHg and ≤170 mmHg (ClinicalTrials.gov: NCT02901704). The results were presented at the China Interventional Therapeutics (CIT) conference in 2023. At six months, the decrease in mean 24-hour ambulatory systolic BP (primary efficacy outcome) was larger in the RDN group (−13.0±12.1 mmHg, n=107) when compared with the sham control group (−3.0±13.0 mmHg, n=110, baseline-adjusted between-group difference −9.4 mmHg, 95% CI: −12.8 to −5.9; p<0.001). In line with previous second-generation sham-Âcontrolled trials, RDN continuously reduced systolic and diastolic BP over 24 hours (“always-on” effect). The procedure was safe; only one patient in the RDN group experienced an access site haematoma which resolved without sequelae.
The Renal Artery DenervatIon Using Radial accesS in Uncontrolled HyperTensioN (RADIUS-HTN) Study is currently enrolling patients in Europe to investigate transradial compared with transfemoral RDN in uncontrolled hypertension despite treatment with 2-5 antihypertensive drugs (ClinicalTrials.gov: NCT05234788). Compared with femoral access, radial access might shorten the hospital stay, increase patient comfort, and reduce vascular complications, thereby further improving the safety profile of the procedure.
SYMAP SYSTEM
The SyMap system (SyMap Medical) utilises electric stimuÂlation of the renal arteries to identify areas whose stimulation elicits an acute increase in systolic BP by ≥5 mmHg (“hot spots”) while avoiding the ablation of parasympathetic nerve fibres (“cold” or “neutral spots”)31. After the ablation, efficacy is confirmed by repeating the initial stimulation32. If systolic BP rises ≥5 mmHg during the repeat stimulation, a second ablation is performed at the same spot. The catheter has a steerable tip within a sheath that can be used to advance or return the catheter while rotating 360 degrees in the sheath32.
The randomised, sham-controlled Sympathetic Mapping/Ablation of Renal Nerves Trial (SMART) for treatment of hypertension evaluated the safety and efficacy of renal mapping and selective RDN in uncontrolled hypertension (office systolic BP 150-180 mmHg) despite standardised antihyperÂtensive two-drug therapy (ClinicalTrials.gov: NCT02761811)32. The trial’s primary efficacy outcomes were (i) the proportion of patients with office systolic BP control (≤140 mmHg, non-inferiority) and (ii) the difference in medication burden, calculated as a drug index (superiority) between the RDN and the sham group, at six months32. Both outcomes had to be met for the trial to be considered positive32. In patients with office systolic BP above target at three months, a standardised intensification of antihypertensive pharmacotherapy was mandated32. The trial results were presented at EuroPCR 2023. A total of 220 patients (87% male, mean age 45 years) were randomised to RDN or a sham procedure at 15 Chinese sites. Office systolic BP control rates were similar between both groups (95% in the RDN vs 93% in the sham group; p=0.429; p<0.001 for non-inferiority). Although the drug index increased in both groups, it rose to a lesser extent in the RDN group (4.4±6.7) compared with the sham group (7.6±10.3, between-group difference −3.3, 95% CI: −5.6 to −0.9; p=0.003 for superiority), indicating the need for fewer drugs, or at least lower doses, following RDN to achieve BP control. Notably, drug adherence was surprisingly high (about 90% at six months) when considering other second-generation RDN trials. There was no difference in the rate of serious adverse events between both treatment groups (RDN 10.8% vs sham 9.2%; p=0.823). One patient in the RDN group developed renal artery stenosis.
The SMART Trial has demonstrated the feasibility and safety of renal mapping and selective RDN. However, future trials will have to investigate the added benefit of selective RDN.
Ultrasound renal denervation
Two catheter systems (Paradise [ReCor Medical]33 and TIVUS [SoniVie]34) that use ultrasound (US) energy for thermal ablation of afferent and efferent nerves are under investigation for RDN (Table 1). Evidence from sham-controlled trials, however, only exists for the Paradise catheter system.
PARADISE RENAL DENERVATION SYSTEM
The Paradise catheter system consists of a cylindrical piezoelectric ceramic transducer located within a low-pressure balloon35. The balloon is pressurised by circulating sterile water, which centres the US transducers within the artery and cools the intima during energy emission35.
The independently powered, multicentre, randomised, sham-controlled Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN) confirmed the BP-lowering efficacy of US-RDN using the Paradise catheter system in patients with mild-to-moderate hyperÂtension without antihypertensive drugs (SOLO cohort) and in patients with resistant hypertension on a fixed-dose, triple combination therapy (TRIO cohort)3637. Compared with the sham group, the trial’s primary outcome, daytime ambulatory systolic BP, was reduced by 6.3 mmHg (95% CI: 3.1 to 9.4; p<0.001) at two months in the SOLO cohort36 and by 4.5 mmHg (95% CI: 0.3 to 8.5; p=0.022) at two months in the TRIO cohort (Figure 1)37. Long-term follow-up of the RADIANCE-HTN SOLO trial indicates that the BP-lowering effect was maintained up to 36 months, since the office BP further decreased and control rates improved, while the number of antihyperÂtensive medications remained stable38.
The RADIANCE II Pivotal Study: A Study of the ReCor Medical Paradise System in Stage II Hypertension (RADIANCE-II) randomised patients withdrawn from antihypertensive medications to RDN (n=150) or a sham procedure (n=74). The study confirmed the findings of the RADIANCE-HTN SOLO trial, by showing clinically relevant reductions in daytime ambulatory systolic BP (baseline-adjusted between-group difference: −6.3 mmHg; 95% CI: −9.3 to −3.2; p<0.001) at two months39. In a patient-level meta-analysis of these three trials comprising 506 patients, the BP reductions two months after US-RDN were consistent across trials, various patient subgroups, and a wide range of hypertension severity (mean difference in daytime BP between RDN and sham control: −5.9 mmHg; 95% CI: −8.1 to −3.8 mm Hg; p<0.001)31. Independent predictors of a larger BP response were higher baseline BP and heart rate, as well as the presence of orthostatic hyperÂtension31. Moreover, US-RDN was safe, as only one periprocedural vasovagal event and one vascular access complication occurred, which were both resolved without further sequelae31.
In contrast, the randomised, sham-controlled RDN on Quality of 24-hr BP Control by Ultrasound in Resistant Hypertension (REQUIRE) Study, conducted in Japan and South Korea, did not meet its primary efficacy endpoint, which was defined as the difference in change in 24-hour systolic BP at three months between the RDN and sham groups. At one month, the reduction in 24-hour systolic BP was significantly greater in the RDN group than in the sham group, but from then on, BP also decreased in the sham group, so that the between-group difference eventually disappeared40. The trial had methodological limitations, including a lack of blinding of the treating physicians, and there was no evaluation to ensure that the patients’ blinding was effectively maintained throughout the trial. The ESC Council on Hypertension and EAPCI consensus paper has classified the trial as not being of the highest quality2. The BP drop in the sham group was likely caused by an uneven intensification of antihypertensive pharmacotherapy41. Moreover, a post hoc analysis from this trial has shown that RDN significantly lowered BP compared with sham treatment in patients with good adherence at baseline42.
In conclusion, US-RDN is a safe and effective treatment for hypertension in a wide range of patients, including those with resistant hypertension. Notably, the RADIANCE-HTN TRIO trial is the only second-generation sham-controlled trial that exclusively enrolled patients with “true” resistant hyperÂtension on a guideline-recommended triple fix combination. The Paradise RDN system has been approved by the FDA for hypertension treatment.
Alcohol-mediated renal denervation
PEREGRINE RENAL DENERVATION SYSTEM
The Peregrine System is currently the only catheter system with published data from a sham-controlled trial43. The Peregrine System Infusion Catheter (Ablative Solutions) enables the infusion of microdoses (0.6 ml per treatment site) of dehydrated alcohol via three extendable ultrathin microneedles into the perivascular space of renal arteries4445. Preclinical models have demonstrated that the alcohol radially distributes in the perivascular space, causing denervation of afferent and efferent sympathetic nerves4446.
An open-label study showing significant BP reductions without major procedural complications in resistant hypertension47 has informed the design of two randomised sham-controlled trials investigating the Peregrine System in the absence (TARGET BP OFF-MED; ClinicalTrials.gov: NCT03503773) or presence (TARGET BP I; ClinicalTrials.gov: NCT02910414) of antihypertensive medications48.
The TARGET BP OFF-MED Trial, which was conducted in 25 centres in Europe and the USA, randomised 106 patients to undergo RDN (n=50) and sham control (n=56) without concomitant medication. The trial was designed as a proof-Âof-concept trial, not powered for efficacy outcomes43. At two months, the change in 24-hour systolic BP was −2.9±7.4 mmHg (p=0.009) and −1.4±8.6 mmHg (p=0.25) in the RDN and sham control group, respectively, without a significant between-group difference (−1.5 mmHg; 95% CI: −4.8 to 1.7; p=0.27)43. The 24-hour diastolic and office BP were also not significantly reduced between baseline and two months43. There were no differences in safety events between groups43. Following the primary outcome collection, antihypertensive drugs were initiated to achieve a target office systolic BP ≤140 mmHg while patients and treating physicians remained blinded to treatment allocation43. After 12 months, patients achieved similar office systolic BP (RDN: 147.9±18.5 mmHg, sham: 147.8±15.1 mmHg; p=0.68), but those in the RDN group required less antihypertensive medications (mean daily defined dose at 12 months: 1.5±1.5 vs 2.3±1.7; p=0.017)43. The COVID-19 pandemic might have influenced the trial outcome, as larger and clinically meaningful BP changes were observed in patients enrolled prior to the start of the pandemic43.
The TARGET BP I Pivotal Trial, which is powered for a change in mean 24-hour ambulatory systolic BP from baseline to three months in patients with uncontrolled hypertension and receiving pharmacotherapy, has completed patient enrolment and is expected to be published in 202448.
Preprocedural imaging
Preprocedural imaging should be performed to screen for anatomical ineligibility criteria, such as severe atherosclerotic renal artery stenosis or fibromuscular dysplasia, and to anticipate anatomical peculiarities (e.g., presence of accessory arteries)2. In one study, angiographic analyses documented accessory renal arteries and renal artery disease in 22% and 9% of RDN patients, respectively49. The ESC/EAPCI consensus statement recommends selecting the imaging modality based on patient characteristics (e.g., obesity), availability, and local expertise2. In general, duplex ultrasonography (DUS) should be considered as a first choice, as it is widely available and does not expose the patient to radiation or contrast. An analysis of the images obtained in the SPYRAL HTN-OFF MED trial suggests that main artery and branch vessel patency were more often evaluable with DUS (88%) than with computed tomography angiography (CTA; 68%) or magnetic resonance angiography (MRA; 29%)50.
Procedural considerations
Figure 2 summarises the key components of a safe and effective RDN procedure. Generally, standard operating procedures for each catheter system and the performance of ≥5 proctored RDN cases with each device are recommended2.
As with other contrast medium-based procedures, adequate patient hydration is recommended. To reduce the amount of contrast dye used, it may be diluted, especially in patients with impaired kidney function. Additionally, alternative contrast media, such as carbon dioxide, may be considered51. Due to nociceptive fibres being transmitted in afferent Aδ and C fibres from the kidney, renal nerve ablation is typically associated with pain. Sufficient analgesia and sedation, for example, using low doses of opioids (e.g., morphine 1-3 mg or fentanyl 1-2 mcg/kg intravenously [IV]) and benzodiazepines (e.g., midazolam 2-3 mg IV or propofol), is a cornerstone of successful RDN2. This not only increases the patient’s comfort, but it might also allow for a safer and more effective procedure, as the patient will be less likely to move during the ablation. Analgesia and sedation require monitoring of vital signs before and immediately after the procedure. According to local legal requirements, a nurse or a physician should be allocated to pain and sedation management. Unfractionated heparin should be administered during the procedure to achieve an activated clotting time of >250 seconds. Moreover, an aspirin loading dose should be administered, followed by 75-100 mg daily for one month post-procedure2.
RDN has a favourable safety profile, and there is no evidence of significant procedure-related safety concerns beyond the risk associated with femoral or radial artery access2. Ideally, a US-guided puncture should be performed. Non-hydrophilic guidewires should be used for the procedure, and the guidewire tip should always be kept in view.
When treating the renal artery, the distribution of sympathetic nerve fibres must be considered. Renal nerves arise from multiple ganglia, splanchnic, and mesenteric nerves and converge to the outer lumen of the renal artery in the distal segments and along the branches. As a result, the lowest number of nerves per quadrant and the shortest lumen-to-nerve distance are found in the distal post-bifurcation segments67. A mean lesion depth of 3.8 mm caused by RF catheters52 would affect more than 90% of the nerves in distal post-bifurcation segments but only 50-60% in the proximal segments (Figure 3)7. Preclinical and clinical studies also support the treatment of branches in addition to the main renal arteries when using RF-RDN5354. Importantly, targeting artery segments within the renal parenchyma is not recommended. However, accessory arteries with vessel diameters eligible for RDN (Table 1) and supplying ≥20% of the renal parenchyma should also be treated. To date, no validated, easily applicable periprocedural clinical indicator of successful renal nerve ablation exists2.

Figure 2. Key components of a safe and effective procedure. ACT: activated clotting time; AE: adverse events; Fr: French; IMA: internal mammary artery; JR: Judkins right coronary; MP: multipurpose; PA: posteroanterior; RDC: renal double curve
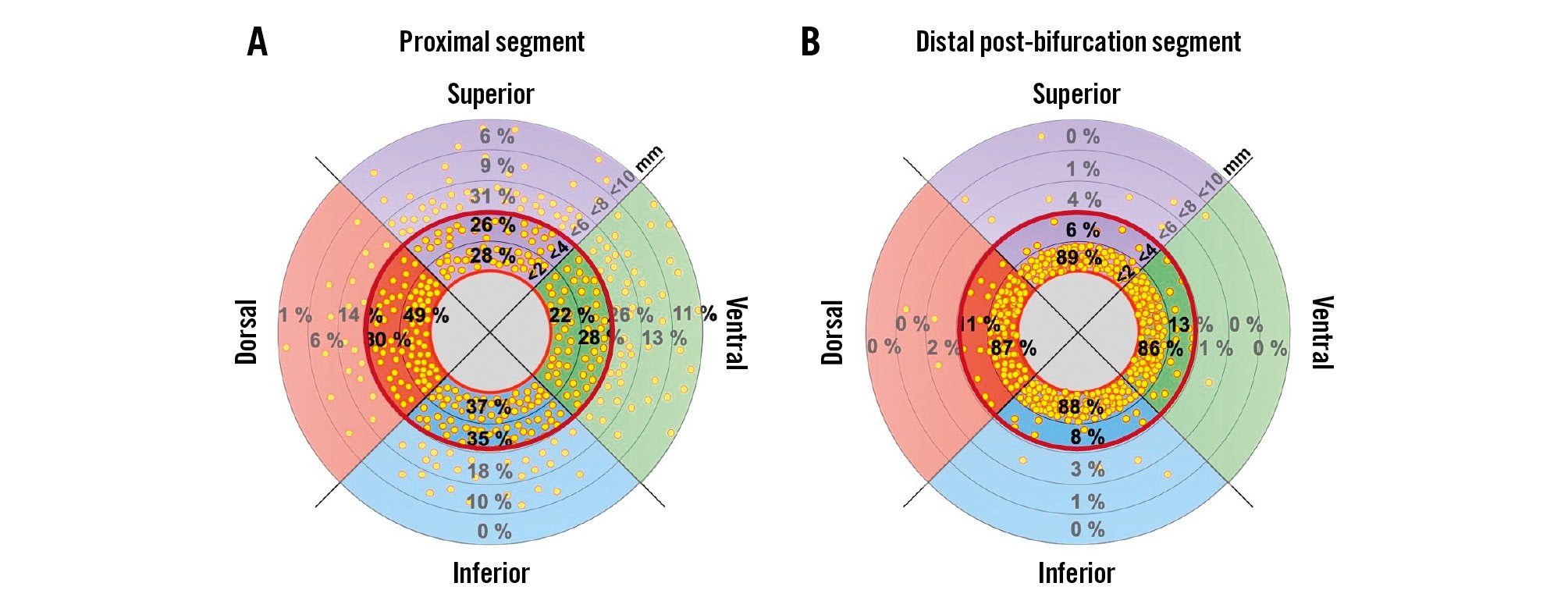
Figure 3. Nerve distribution along the renal artery. The plots show the percentage of nerves in regions within <2, <4, <6, <8, and <10 mm from the renal arteries’ lumen. The red circle represents a penetration depth of 4 mm. Modified with permission from Struthoff et al7.
Recommendations of guidelines and consensus statements
The 2018 ESC/European Society of Hypertension (ESH) hypertension guidelines were based on the first generation of sham-controlled RDN trials and state that “device-based therapies for hypertension are not recommended for the routine treatment of hypertension, unless in the context of clinical studies and randomised controlled trials until further evidence regarding their safety and efficacy becomes available”55. In recognition of the evidence that has become available, several national and international societies and expert groups, including the ESC Council on Hypertension and the EAPCI2, have published consensus statements. Moreover, the ESH recently published its new hyperÂtension guidelines56. Both the ESC/EAPCI consensus statement and the ESH consider RDN a treatment option in patients with resistant hypertension and in those with uncontrolled hypertension despite the use of antihypertensive drug combination therapy, or if drug treatment elicits serious side effects and poor quality of life (Class II recommendation in the ESH guidelines) (Table 2)256. Of note, both the ESC/EAPCI consensus statement and the ESH hypertension guidelines recommend an eGFR ≥40 ml/min/1.73 m2, which was also an inclusion criterion in the sham-controlled trials256. Evidence for patients with more severely decreased kidney function is based on pilot studies and registries57585960. Patient selection should take place in a shared decision-making process, requiring that the patient is well informed about the benefits and limitations of RDN as well as the possible risks associated with the procedure256. The patient must be aware that (i) irrespective of the device used, there is substantial interindividual variability in response across all sham-controlled trials; (ii) as with antihypertensive medications, there is no sensitive and specific predictor of BP response to RDN for an individualised patient selection; and (iii) the primary aim is to reduce BP, and most patients will require further antihypertensive medications. In the second-generation RDN trials, the BP reductions varied between 9.0 mmHg and 11.0 mmHg for office systolic BP and between 4.7 mmHg and 8.5 mmHg for 24-hour systolic BP in the RDN groups (Figure 1).
Table 2. Key statements on RDN in the 2022 ESC/EAPCI consensus statement and 2023 ESH hypertension guidelines.
| 2022 ESC/EAPCI consensus statement | 2023 ESH hypertension guidelines* | |
|---|---|---|
| RDN in uncontrolled hypertension… | May be a possible treatment option for patients unable to tolerate antihypertensive drugs in the long term or patients who express a preference to undergo RDN | Can be considered as a treatment option if drug treatment elicits serious side effects and poor quality of life (COR II, LOE B) |
| RDN in resistant hypertension… | May be used | Can be considered as a treatment option (COR II, LOE B) |
| Secondary hypertension | Secondary causes of hypertension should be excluded | Secondary causes of hypertension should be excluded |
| Lower eGFR threshold | ≥40 ml/min/1.73 m2 | ≥40 ml/min/1.73 m2 |
| Centre requirements | RDN should be performed at experienced centres with multidisciplinary hypertension teams and a hypertension outpatient clinic, inpatient ward, radiology division, hormone testing, clinical laboratory, catheterisation laboratory, coronary care or intensive care unit, and access to an emergent vascular surgery facility, either onsite or remote. Procedures should only be performed by a highly skilled interventionalist with experience in renal artery interventions | RDN should only be performed in experienced and specialised centres (COR I, LOE C) with an established multidisciplinary hypertension team |
| Patient preference | The decision-making process should incorporate the preference of a well-informed patient | Patient selection should take place as part of a shared decision-making process after the patient has received objective and complete information (COR I, LOE C) |
| * The 2023 ESH guidelines applied new criteria for grading the level of evidence. Level of evidence “A” was only considered if cardiovascular outcome data were available. COR: class of recommendation; EAPCI: European Association of Percutaneous Cardiovascular Interventions; eGFR: estimated glomerular filtration rate; ESC: European Society of Cardiology; ESH: European Society of Hypertension; LOE: level of evidence; RDN: renal denervation | ||
Open questions
Although US- and RF-RDN have been proven to safely reduce BP, there are still several unanswered questions. Firstly, there is neither a specific predictor of BP response to RDN nor a marker for successful RDN. Secondly, unlike first-line antihypertensives, there are no cardiovascular outcome trials for RDN. Due to the low residual risk nowadays and as confounding is likely, an outcome trial for RDN cannot be expected in the near future2, although it is undeniably desirable. However, BP decreases represent a well-Âestablished surrogate for cardiovascular outcome reductions, and there is reason to believe that RDN-associated BP changes do not provide similar benefits. An analysis of the Global SYMPLICITY Registry showed that an increase in time in the target range (office systolic BP <140 mmHg and 24-hour systolic BP <130 mmHg) following RDN was associated with fewer major cardiovascular events61. Of note, commonly used and guideline-recommended BP-lowering therapies, including exercise, metabolic surgery, and several drugs, such as mineralocorticoid receptor antagonists, clonidine, moxonidine, doxazosin, minoxidil, and hydralazine, have not been supported by cardiovascular outcome trials in hypertension. Thirdly, well-designed cost-effectiveness studies for RDN are lacking.
Conclusions
The RF and US catheter systems investigated in several high-quality trials have proven safe and effective in lowering BP through RDN. While new technologies continue to be investigated, RDN has become an established and guideline-recommended treatment option for hypertension. To ensure optimal and safe treatment, RDN should only be performed at specialised centres with multidisciplinary hypertension teams (Central illustration). In the decision-making process, the preference of a well-informed patient should be at the forefront.
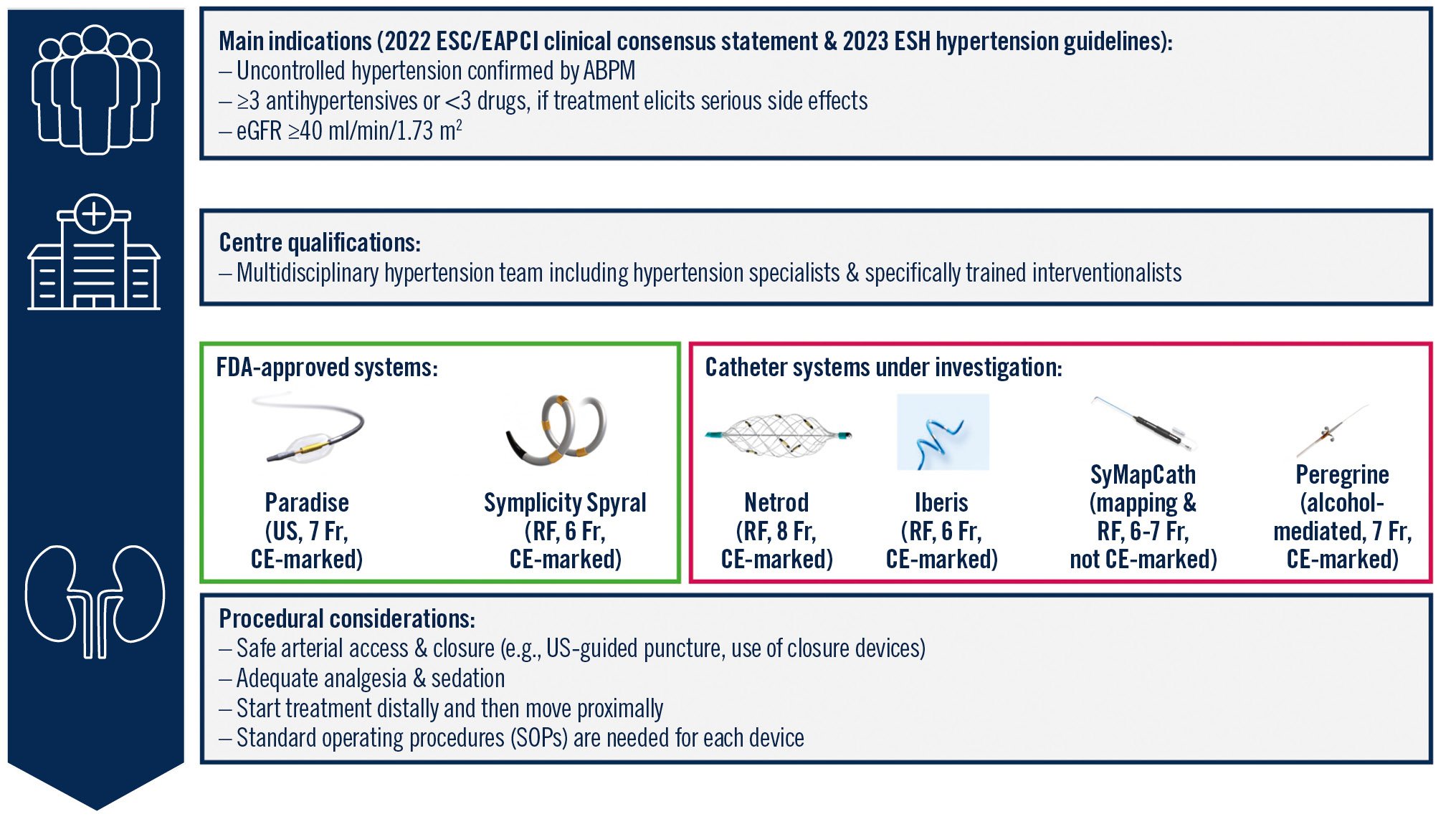
Central illustration. Recommendations for renal denervation in the management of hypertension. ABPM: ambulatory blood pressure monitoring; CE: European conformity; EAPCI: European Association of Percutaneous Cardiovascular Interventions; eGFR: estimated glomerular filtration rate; ESC: European Society of Cardiology; ESH: European Society of Hypertension; FDA: U.S. Food and Drug Administration; Fr: French; RF: radiofrequency; US: ultrasound
Conflict of interest statement
L. Lauder has received speaker honoraria from AstraZeneca, Medtronic, Pfizer, and ReCor Medical. D.E. Kandzari has received institutional research and grant support from Medtronic and Ablative Solutions; personal consulting honoraria from Medtronic and Ablative Solutions; and holds equity in BioStar Ventures, but none related to Ablative Solutions. T.F. Lüscher has received, outside of this work, educational and research grants from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Medtronic, Novartis, Novo Nordisk, Sanofi, Servier, and CSL Vifor; and honoraria from Ablative Solutions, Daiichi Sankyo, Menarini Foundation, Novartis, Novo Nordisk, and Pfizer. F. Mahfoud is supported by Deutsche Gesellschaft für Kardiologie (DGK), Deutsche Forschungsgemeinschaft (SFB TRR219), and Deutsche Herzstiftung; he has received scientific support (to the institution) from Medtronic and ReCor Medical; and speaker honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Inari, Medtronic, Merck, and ReCor Medical.

