Abstract
Aims: Renal denervation is a potential new treatment therapy for resistant hypertension. However, anatomic vascular variations might complicate the procedure and increase complications. A radial approach offers easier renal artery access and is associated with a decreased risk of vascular access complications.
Methods and results: We describe the first-in-man use of a radial access ultrasound-based renal denervation device (Radiance™; ReCor, Palo Alto, CA, USA) in two patients with therapy-resistant hypertension. Both procedures were uneventful and resulted in significant blood pressure reductions at three-month follow-up (mean office blood pressure drop [–40/–29 mmHg]; mean ambulatory blood pressure drop [–11/–8 mmHg]).
Conclusions: Radial access renal artery denervation using the Radiance catheter appears to be safe and effective and should be considered an alternative option in patients referred for renal denervation.
Introduction
Renal denervation using a transfemoral approach has recently been studied as a potential new treatment strategy for therapy-resistant hypertension with a favourable safety profile1-3. However, anatomical variations in renal artery take-off as well as femoral access complications have been raised as potential concerns complicating the procedure4. Current-generation devices require the use of 6 to 8 Fr femoral sheaths, and some of the more bulky devices on the market might make renal artery cannulation difficult in cases of more challenging anatomy.
We describe the first-in-man use of a radial access ultrasound-based renal denervation device (Radiance™; ReCor , Palo Alto, CA, USA). The 5 Fr 145 cm Radiance™ catheter is a second-generation device based on further developments to the 6 Fr Paradise™ catheter and allows energy delivery in 10 seconds per ablation. The system utilises a cooled balloon catheter (Figure 1) with a self-centred piezoelectric ultrasound transducer, which enables controlled, uniform and circumferential energy delivery without direct wall contact. The ultrasound energy consists of high-frequency sound waves (rapid mechanical oscillations). The sound waves pass through the surrounding cooling fluid of the balloon and generate heat in the surrounding tissue with the goal of causing nerve damage. The catheter is currently available with balloon sizes of 5 and 6 mm. Of interest, the associated generator automatically recognises the connected catheter and adjusts energy delivery to the specific balloon size of the catheter (Figure 2). The ReCor clinical trial programme assessing the safety and efficacy of the Paradise and Radiance catheters currently consists of the completed first-in-man feasibility REDUCE study, the French post-market REALISE study and the currently ongoing multicentre ACHIEVE study, which is at present completing enrolment.
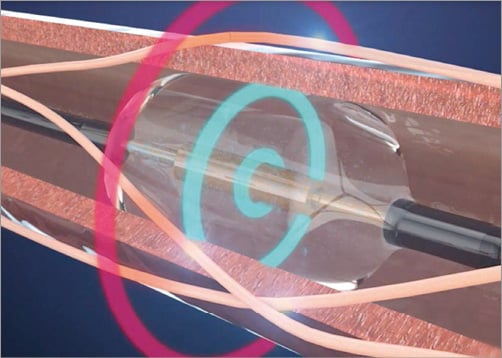
Figure 1. The cooled balloon catheter with a self-centred piezoelectric ultrasound transducer enables controlled, uniform and circumferential energy delivery without direct wall contact.
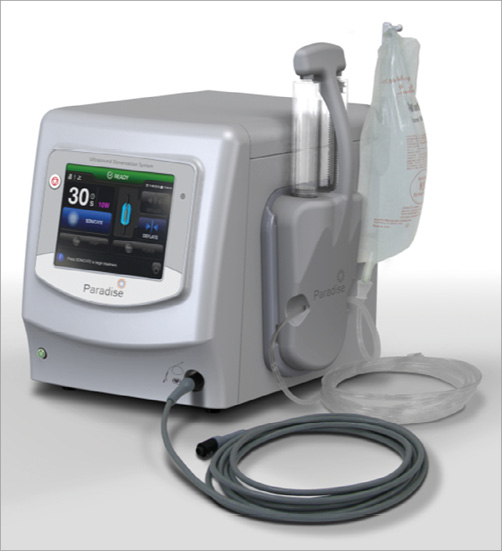
Figure 2. Paradise® renal denervation generator.
Case report
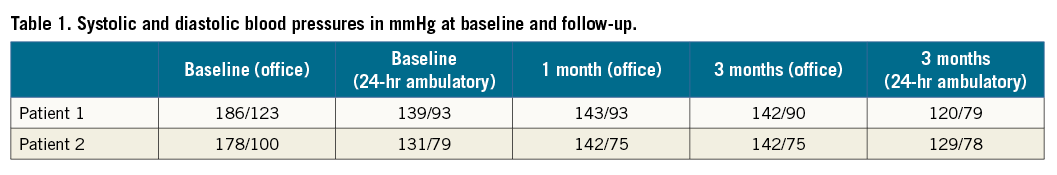
Two patients were treated on the 24th of January 2014 at the Thoraxcenter, Rotterdam, The Netherlands. The first patient was a 75-year-old male with hypercholesterolaemia, a BMI of 29 kg/m2, mild aortic valve stenosis and permanent atrial fibrillation. Renal function was normal with an eGFR of 64 ml/min. He was on a stable antihypertensive drug regimen consisting of three antihypertensive drugs at the maximum tolerated dosage and had an intolerance for calcium antagonists, and average baseline office blood pressure was 186/123 mmHg. The 24-hr ambulatory blood pressure recording showed a mean average of 139/93 mmHg. The second patient was a 55-year-old male with a history of smoking, hypercholesterolaemia, and coronary artery disease with a previous inferior myocardial infarction. BMI was 32 kg/m2 and creatinine clearance was 84 ml/min. Average office blood pressure was 178/100 mmHg along with a value of 131/79 mmHg measured using 24-hr ambulatory blood pressure monitoring. He was on a stable regimen of five antihypertensive drugs.
After exclusion of secondary causes of hypertension, both patients were considered eligible for renal sympathetic denervation. Despite signs of white coat hypertension, patient 2 was considered a candidate for the treatment due to his long-standing hypertension despite five antihypertensive drugs at the maximum tolerated dosage, end-organ damage with mild hypertensive nephropathy and concentric left ventricular hypertrophy.
Both procedures were performed under local anaesthesia. A 6 Fr introducer sheath was inserted into the left radial artery (Moving image 1). A radial cocktail for preventing radial artery spasm was administered using heparin (5,000 units), nitroglycerine (200 mcg) and verapamil (5 mg). A 0.038 inch hydrophilic wire (Terumo Corp., Tokyo, Japan) was used to access the descending aorta. A 6 Fr pigtail catheter was used to make an aortogram and confirm renal artery anatomy. Using a standard 180 cm exchange wire, the pigtail was exchanged for a 6 Fr ADROIT™ JR4 guiding catheter of 125 cm (Cordis, Johnson & Johnson, Warren, NJ, USA), which easily allowed renal artery access. Using a Hi-Torque BHW wire (190 cm) (Abbott Vascular, Santa Clara, CA, USA) the renal artery was cannulated. The Radiance™ catheter and flushing cartridge were connected to the generator. Once connected, the generator recognised the catheter and the preparation process was initiated wherein sterile water was circulated to remove air from the closed system. Once this process was finished, the energy delivery was verified by holding the device under water, which allows the identification of the ultrasound waves. Once prepared and tested, the monorail Radiance™ catheter was loaded. Using a standard Y-connector, the catheter passed easily through the guide and accessed both renal arteries in a more natural way than is usually the case via a transfemoral approach. After confirmation of the position of the catheter just before the first large bifurcation, the 10-second ablation commenced by a click on the screen of the generator or footswitch. Two ablations per artery were performed. Periprocedural pain medication consisted of 3 mg midazolam and 75 mcg of fentanyl, which allowed sufficient comfort for both patients. Post-procedural angiograms showed no signs of thrombus or dissection. The average procedure time was 55 minutes (radial access to close) and contrast use was 70 ml for both procedures. A TR Band™ (Terumo) was used for radial artery access-site closure. The post-procedural course was uneventful with a normal Barbeau test confirming renal artery patency. No post-procedural pain, haematoma or patient discomfort was present. Both patients could be discharged the next day. Currently, three-month follow-up is available for both patients. Patient 1 showed a decrease of –44/–33 mmHg in office systolic blood pressure along with a drop of –19/–14 mmHg in 24-hr ambulatory blood pressure. Patient 2 showed an office-based blood pressure drop of -36/-25 mmHg at three months without any significant change in 24-hr ambulatory blood pressure (–2/–1 mmHg) (Table 1). Both patients reported a normal hand function. Follow-up imaging using MRA showed no signs of luminal narrowing, and renal function remained unchanged (Δ eGFR –1 ml/min in patient 1 and 0 ml/min in patient 2).
Discussion
The present cases demonstrate the feasibility, safety and efficacy of renal artery denervation using a transradial approach. Previously reported periprocedural complications in renal artery denervation were to a great extent due to vascular access complications (up to 50% in a recently presented study). In contrast, transradial catheterisation has been associated with improved patient comfort, decreased length of stay and hospital costs, and decreased access-site complications5.
For the present cases the left radial was chosen as first choice access site because of the shorter length needed to reach the renal arteries and avoidance of the right subclavian/aortic root arch. Another practical advantage is the current availability of 125 cm guiding catheters, allowing sufficient length to access the renal artery through a left radial approach. Additionally, the BHW guidewire offers sufficient support to position the balloon-based device easily in the renal artery without having a hydrophilic coating that could increase the risk for dissections in the peripheral renal artery bed.
Failure of the Symplicity™ device (Medtronic, Minneapolis, MN, USA) to generate sufficient renal nerve damage has been hypothesised to be one of the main reasons for the negative results of the recently published SYMPLICITY HTN-3 trial2,6. The ReCor system offers several features that might increase the technical success, e.g., a higher penetration depth and circumferential energy delivery matching current insights on renal nerve location, namely within the first 6 mm instead of 2 mm from the arterial lumen; a higher level of post-procedural norepinephrine reduction as compared to data on the Symplicity system; and a cooling balloon having no direct contact of the energy source with the arterial wall, which preserved the integrity of the endothelial lumen in recent porcine models7.
Only two ablations were performed per renal artery, which was justified by pathophysiological evidence demonstrating a lack of additional effect on reducing norepinephrine levels with two as compared to three or more ablations with the ReCor device7. Given the short ablation time, together with the apposition of the balloon with the artery wall, and a little slack in the catheter, movement of the energy source due to pain and/or respiration did not occur with the technique described above.
Since renal arteries on most occasions have a somewhat downward take-off, a radial approach is more natural and allows easier renal artery access, lowering the need for stiffer guiding catheters and guidewires, as well as lower contrast use and radiation time. Despite these advantages, the preferred access site should be evaluated on a per-patient basis taking into account radial artery patency and sufficient ulnar collateral circulation as well as significant upper extremity arterial disease. Another limitation of the current version of the device is that its use is limited to arteries ranging from 4.5 to 6 mm, since 4, 7 and 8 mm balloons are not currently available.
In conclusion, we believe radial access renal artery denervation should be considered a valuable alternative option in patients with more complex renal artery anatomy, lower extremity peripheral vascular disease or higher risk of bleeding.
| Impact on daily practice Anatomical variations in renal artery take-off as well as femoral access complications have been raised as potential concerns complicating the renal denervation procedure. Unfavourable renal artery anatomy in combination with a suboptimal device profile might spark the use of back-up manoeuvres, using supportive back-up wires and mother-in-child catheters with a subsequent exponential increase in the risk of complications. In the present special report we demonstrate that radial access renal artery denervation using the 5 Fr ReCor Radiance™ catheter can be a valuable alternative option in patients with more complex renal artery anatomy, lower extremity peripheral vascular disease or higher risk of bleeding. |
Acknowledgements
We are grateful to Rakesh Ramdhan for his help in preparing the moving image.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Radial renal denervation procedure using the ReCor Radiance™ catheter.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1.

