Abstract
Aims: Enhanced vascular ageing is associated with elevated central pulse pressure (cPP), an independent predictor of cardiovascular (CV) events. Although antihypertensive treatment strategies are effective, high residual CV risk remains indicative of advanced and largely irreversible vascular damage. Renal denervation (RDN) has been shown to reduce blood pressure (BP) to various extents in patients with treatment-resistant hypertension (TRH). We hypothesised that cPP predicts BP reduction after RDN.
Methods and results: Sixty-three patients with true TRH underwent catheter-based RDN using the Symplicity Flex™ catheter and were followed for six months. At baseline, cPP was assessed by pulse wave analysis (SphygmoCor™). Patients were stratified according to their median cPP (55 mmHg), and called “low cPP” (below the median) or “high cPP” (above the median). Office BP reduction six months after RDN was greater (–22±19/–13±11 vs. –12±20/–5±13 mmHg, p=0.038/0.014) and 24-hr ambulatory blood pressure (ABP) reduction tended to be greater (–11±13/–8±10 vs. –3±18/–4±10 mmHg, p=0.070/0.112) in patients with low cPP compared to those with high cPP. Only cPP (β=0.687, p=0.001) and baseline systolic BP (β=–0.564, p<0.001) were independent determinants of office systolic BP reduction after RDN.
Conclusions: Our data suggest that cPP, indicative of the degree of large arterial stiffening, may be helpful to identify responders to RDN.
Introduction
Vascular ageing is associated with functional and structural alterations, which are exaggerated in the presence of several cardiovascular (CV) risk factors and CV diseases such as hypertension. Changes in the macrovasculature and microvasculature are herein deeply interrelated. Microvascular structural alterations (i.e., increased wall:lumen ratio and rarefaction of small arteries) are a major factor for an increase in mean blood pressure (BP), which, in turn, increases shear stress and hence arterial stiffness of large arteries indicated by an increased central pulse pressure (cPP)1. Hence, increased pulsatile pressure subsequently damages small arterioles, i.e., induces and aggravates microvascular damage and vice versa. Pathophysiologically, central pressure in the aorta, which is actually the perfusion pressure to key organs, rather than the pressure in the arm, may provide more relevant prognostic information. Indeed, several studies have shown that cPP is more strongly related to target organ damage such as left ventricular mass (LVM)2, and reduction of cPP was strongly correlated to the change in LVM, although no correlation was found with peripheral PP3. Moreover, cPP more accurately predicts all-cause and CV mortality compared to peripheral PP4, and, in the Conduit Artery Function Evaluation (CAFE) study, a Cox proportional hazards modelling showed that cPP was significantly associated with a post hoc defined composite outcome (total CV events/procedures and development of renal impairment)5. In the latter and other studies, it was demonstrated that antihypertensive drugs affect central as opposed to peripheral BP differently5,6. In general, the dissociation between central and brachial BP has been observed to be greater at higher baseline BP levels, regardless of the treatment strategy used7.
Management of treatment-resistant hypertension (TRH) remains a major challenge, in particular achieving BP control. Since its introduction a few years ago, renal denervation (RDN) has emerged as an interventional approach to reduce BP in TRH8-10. RDN leads to a decrease of renal efferent sympathetic activity and afferent sensory signalling to the central nervous system and, hence, towards key organs, including the vasculature, in particular the small resistance vessels11,12. However, BP response rates (typically defined as office systolic BP ≥10 mmHg) due to RDN vary9,13,14, and hence one of the most critical and important issues is to identify a reliable predictor of BP response. Previous analyses have focused mainly on clinical characteristics and technical aspects and failed, with the exception of baseline systolic BP9,15, which per se is related to the amount of BP reduction according to Wilder’s law16, and regression to the mean effect.
In the present analysis, we therefore focused on a more pathovascular approach, namely that cPP as a “hypertensive disease marker” integrates the cumulative burden of various CV risk factors and, hence, their induced damage on the vascular system.
Methods
STUDY COHORT
In this study, 63 patients with TRH (office BP ≥140/90 mmHg and 24-hr ABP ≥130/80 mmHg, despite treatment with at least three antihypertensive drugs including a diuretic)17 who underwent RDN were consecutively included if central haemodynamics were measurable in high quality (SphygmoCor™; AtCor Medical, West Ryde, NSW, Australia). Patients had to be on a stable drug regime (i.e., without change in dose or medication) for at least four weeks prior to study inclusion. In line with the recent position papers of the European Society of Hypertension18 and the European Society of Cardiology19, the main exclusion criterion was renal artery anatomy that was ineligible for treatment (main renal arteries <4 mm in diameter or <20 mm in length, haemodynamically or anatomically significant renal artery abnormality or stenosis in either renal artery, history of prior renal artery intervention including balloon angioplasty or stenting). In addition, a secondary cause of hypertension (except treated obstructive sleep apnoea syndrome) was an exclusion criterion, and estimated glomerular filtration rate (eGFR) had to be ≥15 ml/min/1.73 m2.
The study protocol was approved by the local ethics committee (University of Erlangen) and was performed according to the Declaration of Helsinki and “Good Clinical Practice” (GCP) guidelines. Written informed consent was obtained from all patients before study entry. The study was registered at www.clinicaltrials.gov (ID: NCT01687725).
CATHETER-BASED RENAL DENERVATION
For RDN, the femoral artery was accessed with a standard endovascular technique. A radiofrequency catheter (Symplicity Flex™ RDN System; Medtronic Inc., Santa Rosa, CA, USA) was advanced in each renal artery guided by angiography. As described in detail previously8, at least four radiofrequency ablations (energy delivery for 120 seconds each), controlled and regulated by a radiofrequency generator, were applied longitudinally and rotationally within the lengths of each renal artery. Patients received 5,000 IU heparin, and diffuse visceral pain during the procedure was managed with anxiolytics and narcotics.
OFFICE AND 24-HR AMBULATORY BLOOD PRESSURE
Office BP was measured initially in both arms after five minutes of rest in a sitting position with an oscillometric device (Dinamap Pro 100V2; Criticon, Norderstedt, Germany). Subsequent BP measurements were performed on the arm with the higher BP readings and the average of three measurements was taken. 24-hr ABP measurements were performed with validated automatic portable devices.
CENTRAL HAEMODYNAMICS
In the supine position, central haemodynamics using the SphygmoCor System were assessed prior to RDN as described in detail20. In brief, first brachial BP was recorded at the dominant arm with an oscillometric device (Dinamap Pro 100V2; Criticon), and the last three measurements were averaged. Subsequently, radial artery waveforms were sampled in the same arm (gently hyperextended wrist) by a non-invasive technique, calibrated to the mean arterial pressure and diastolic BP. The radial artery waveform was averaged from single waveforms recorded consecutively for 10 seconds. Corresponding central (aortic) waveforms were then automatically synthesised from the radial artery waveform by a built-in validated transfer function. From the derived central waveform, data are given for central systolic, diastolic BP and hence central PP. A good agreement between non-invasively and invasively assessed BP has been shown repeatedly20-22. Duplicate recordings included in the analysis were of high quality, defined as quality index >80% (based on an in-device algorithm).
MEDICATION ADHERENCE
Urine samples were routinely collected at baseline and six months after RDN. Toxicological urine analyses of antihypertensive compounds or metabolites were carried out retrospectively from stored spot urine samples. For detail, see Jung et al23. In brief, analysis was carried out using a liquid chromatography-mass spectrometry (LC-MS/MS) system from Agilent (Waldbronn, Germany). For hydrochlorothiazide, furosemide and xipamide, the negative electrospray interface (ESI) mode was used; all other compounds were analysed in positive ESI mode. Data evaluation was performed using the Agilent MassHunter software (B 0.601). Identification was achieved based on comparison with the results from analysis of a blank urine and a urine extract containing a reference substance of all target compounds in low concentrations. A deviation of ±0.1 min of the expected retention time and a quantifier/qualifier ratio ±20% of the expected ratio were required.
STATISTICAL ANALYSES
All analyses were performed using IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA). Following our hypotheses, patients were categorised according to the median cPP, and called “low cPP” (below the median) or “high cPP” (above the median). The normality of data distribution was evaluated using the Kolmogorov-Smirnov test. Normally distributed data are presented as mean±standard deviation (SD), and non-normally distributed data as median and interquartile range. They were compared by paired and unpaired Student’s t-tests, Wilcoxon and McNemar tests, as appropriate. Change in medication adherence was tested with the chi-square test. Univariate correlations were assessed using the Pearsons’s correlation coefficient. Multiple stepwise regression analysis was conducted to determine whether cPP was related to the BP reduction independently of possible confounders. A two-sided p-value of <0.05 was considered statistically significant.
Results
CLINICAL CHARACTERISTICS
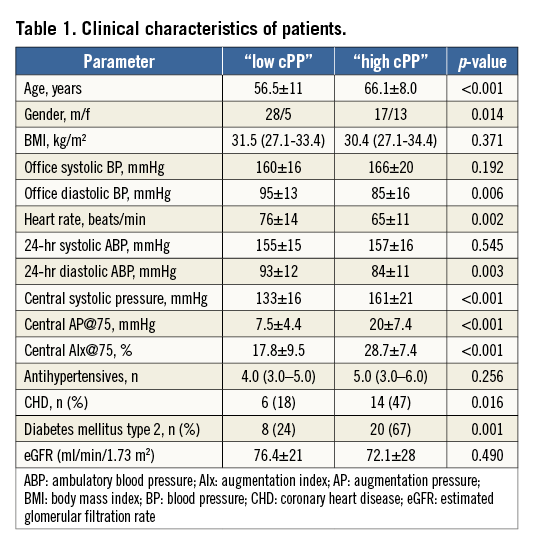
The baseline characteristics of patients divided into low cPP and high cPP are depicted in Table 1. Patients with high cPP were older, had a lower heart rate (HR) and lower diastolic BP than patients with low cPP. There was no difference in body mass index (BMI) and eGFR between the groups, whereas the prevalence of diabetes and coronary heart disease (CHD) differed (Table 1).
BLOOD PRESSURE
Six months after RDN, office BP was reduced in both cPP groups (low cPP: 160±16/95±13 versus 137±16/82±11 mmHg, p<0.001/<0.001; high cPP: 166±20/85±16 versus 154±26/80±13 mmHg, p=0.003/ 0.049), but the reduction was significantly greater in patients with low rather than high cPP (–22±19/–13±11 versus –12±20/–5±13 mmHg, p=0.038/0.014) (Figure 1). In patients with available 24-hr ABPM at baseline and after six months (n=60), 24-hr ABP was significantly reduced in patients with low cPP (155±15/93±12 versus 144±15/ 86±10 mmHg, p<0.001/<0.001), but not in those with high cPP (157±16/84±11 versus 154±23/81±12 mmHg, p=0.326/0.059). In accordance with the results found with office BP measurements, 24-hr ABP reduction tended to be greater in patients with low rather than high cPP (–11±13/8±10 versus –3±18/–4±10 mmHg, p=0.070/0.112) (Figure 2).
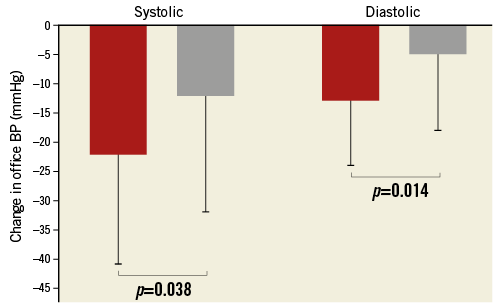
Figure 1. Systolic (left columns) and diastolic (right columns) office blood pressure (BP) reduction after renal denervation (RDN) in patients with low (red) versus high (grey) central pulse pressure (cPP).
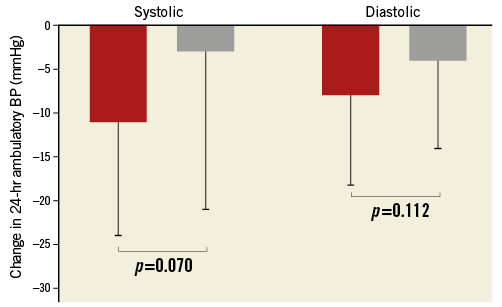
Figure 2. Systolic (left columns) and diastolic (right columns) 24-hr ambulatory blood pressure (BP) reduction after renal denervation (RDN) in patients with low (red) versus high (grey) central pulse pressure (cPP).
MEDICATION ADHERENCE (n=59)
There were no significant differences in toxicological detected antihypertensive drugs both at baseline (4.0 [3.0-5.0] versus 5.0 [3.0-6.0], p=0.256) and six months after RDN (4.0 [3.0-5.0] versus 4.0 [3.0-6.0], p=0.370) between the low and high cPP subgroups. These toxicological data indicate that at baseline in both subgroups 71% of patients were reaching a compliance rate >80% (representing used compliance criteria in randomised medication trials). In patients with full compliance to drug therapy (>80% of prescribed drugs detected in the urine) office BP was more reduced in patients with low cPP than in those with high cPP (–22±21/–13±13 versus –10±19/–3±14 mmHg, p=0.049/0.020).
RENAL FUNCTION
Renal function, assessed by eGFR according to the Modification of Diet in Renal Disease study formula, remained unchanged in both subgroups (low cPP: 76.4±21 versus 76.0±22 ml/min/1.73 m2, p=0.846; high cPP: 72.1±28 versus 70.1±30 ml/min/1.73 m2, p=0.243). None of the patients developed a doubling of creatinine or required dialysis.
REGRESSION ANALYSES
To determine the influence of potential confounders on office systolic BP reduction after RDN, multiple linear regression analyses were performed. Neither age (β=–0.258, p=0.072) nor gender (β=–0.170, p=0.172) emerged as an independent predictor. Ethnicity was not entered since all were Caucasians. Concomitant diseases (CHD: β=–0.050, p=0.687; diabetes mellitus: β=–0.035, p=0.803) and HR (β=0.128, p=0.366) were also not related to the office systolic BP reduction. Only cPP (β=0.687, p=0.001) and baseline systolic BP (β=–0.564, p<0.001) were independent determinants of office systolic BP reduction after RDN.
Discussion
A challenging problem regarding RDN is the identification of the optimal candidate for RDN. Published rates of BP response due to RDN, arbitrarily defined as BP reduction of at least 10 mmHg, differ widely9,13,14. Hence, in the various published clinical studies on the effects of RDN, analyses have been performed to delineate predictors of BP response. Most of these approaches have focused on patient factors and potential biomarkers9,24,25. Indeed, previous studies have suggested that cardiac baroreflex sensitivity (BRS), levels of soluble fms-like tyrosine kinase-1 (sFLT-1), intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) predict the response to RDN26,27. However, concerns have been raised as to whether antihypertensive medication has a direct relationship with inflammatory biomarker levels, including sFLT-128. At best, such a pathophysiological link appeared to be rather weak29.
Overall, it may be difficult or even impossible to simplify the BP response to a single biomarker, since a large proportion of patients with TRH have several additional comorbidities indicating a very heterogeneous patient population per se.
In contrast, arterial stiffness in general and aortic stiffness in particular, known to be markedly increased in patients with TRH30, can be considered as a measure of the cumulative long-lasting burden of all identified and non-identified CV risk factors with ageing on the arterial tree. Non-modifiable factors such as age, gender31, genetic markers32, and also several thorough fluctuating, but in the long term stable factors, such as level of BP, high salt intake33 or metabolic abnormalities34 (representing “snapshots” at the given time point), have been proposed as impacting on vascular structural remodelling, and hence CV risk. Besides arterial stiffness, the timing and magnitude of pressure wave reflections impact on the level of central BP35-37. Thus, the damage to the arterial wall, reflecting the integrated damage, has been proposed as a “hypertensive disease marker”38.
Taking these conceptual points into account, we stratified our patients according to the median cPP, and the main finding of our study is that BP reduction following RDN assessed either by office BP or by 24-hr ABPM is uniformly greater in patients with low cPP, indicative of a lower degree of damage of the arterial vasculature. Conversely, our data suggest that, in patients with high cPP who showed a modest if any BP reduction at all, BP may have established vascular damage to such a degree that it precludes any reversal of arterial damage, at least in this time period of six months. Accordingly, it has been shown that BP reduction after RDN is less pronounced in patients with isolated systolic hypertension (ISH) compared to patients with systolic and diastolic hypertension39. In the SYMPLICITY HTN-3 trial, similar results have been observed (unpublished data).
Improvement in arterial function in small resistance vessels and large arteries can occur due to changes in vasoconstrictive tone and/or reversed vascular damage, i.e., remodelling. However, changes of the vasoconstrictive tone can only occur if the arterial wall still has the ability to vasodilate, which is not the case in severely damaged arteries. Thus, changes of sympathetic activity known to influence vasoconstriction and vasodilation may only have the potential to decrease BP by vasodilation in the short term if the wall is still able to react and vasodilate. Obviously this ability is lost in patients with TRH characterised by high cPP.
This concept is supported by a previous study which compared normotensive to hypertensive subjects. The vascular reactivity to norepinephrine administration was exaggerated in hypertensive patients and involved not only small resistance vessels but also large arteries, hence decreasing their conducting and buffering function40. Moreover, it was shown that arterial distensibility was diminished in response to sympathetic activation41,42, and vice versa, that removal of adrenergic tone by anaesthesia of the brachial plexus and the spinal cord results in markedly increased distensibility of the radial and femoral artery43. Accordingly, in an animal model it was shown that thoracic sympathetic denervation improved both structural and functional remodelling of the aortic wall44.
Finally, non-modifiable risk factors of arterial stiffness, e.g., age, can be integrated into this concept. Age is the main modulator of arterial properties and the damage to the arterial wall is also integrated into this concept45. Likewise, HR, another factor of arterial damage, is regarded as a defining factor of the “timing synchronisation” of the forward and backward travelling waves46. Hence, we are not surprised that in the regression analyses both age (β=–0.258, p=0.072) and HR (β=0.128, p=0.366) were not related to the BP response, since their effect is reflected by the vascular damage to the arteries. In contrast, cPP (β=0.687, p=0.001), in our opinion a valid marker of vascular damage, was identified as an independent determinant of BP response due to RDN.
One potential confounder never rigorously analysed in the RDN trials published so far is adherence to medication. It was reported that in a selective cohort (tertiary) centre about 50% of patients with TRH were non-adherent, based on toxicological urine analyses23. We therefore attempted to control for this important confounder and determinant. To overcome this shortcoming, we have now for the first time assessed whether findings are biased by medication adherence. It is noteworthy to mention that toxicological urine analyses were carried out retrospectively using already sampled urine, hence ruling out a possible bias in medication adherence due to awareness in doing so. Moreover, in contrast to previous study, analyses were not carried out until after each patient had given his/her consent. Toxicological detected antihypertensive drugs both at baseline and six months after RDN did not differ in patients with low or high cPP, and the grade of adherence did not change significantly after RDN in both subgroups. It is also noteworthy to mention first that, in both cPP groups, 71% of patients were reaching an adherence rate >80% (representing the compliance criteria used in randomised medication trials), and secondly that our results did not change if we restrict the analyses to those with an adherence rate >80%.
Shortcomings related to technical aspects, which hampered the previously published SYMPLICITY HTN-3 trial47,48, are unlikely since the team at our centre has over five years of experience in conducting RDN, has been properly trained and has used the same device (Symplicity Flex catheter) in over 120 patients. However, although our study is an observational follow-up study, which lacks a randomised control group, it is of high quality since we used blind endpoint evaluation and controlled for non-adherence. Additionally, the sample size of our single-centre cohort is rather small and needs corroboration by other trials; however, single-centre trials have the advantage of reducing variability in clinical parameters, such as BP response, which is inherent in multicentre trials. Since we did not assess cPP immediately after RDN, we cannot delineate whether acute changes of cPP predict the response of BP after six months.
In conclusion, our data suggest that central PP, the pulsatile component of BP, indicative of the degree of large arterial stiffening, predicted BP response after RDN. This result is in line with the concept that arterial stiffening reflects vascular damage due to hypertension, comorbidities and concomitant CV risk factors. The biological age of arterial stiffening may be helpful to identify responders to RDN.
| Impact on daily practice To identify a reliable clinical predictor of BP response after RDN we used a pathophysiological approach, namely cPP, since an improvement of arterial function (and thereby BP) is based on the ability to vasodilate, which may not be the case in severely damaged arteries. In doing so, our data suggest that cPP, indicative of the degree of large artery damage, may be helpful to identify possible candidates for RDN. Noteworthy, by analysing medication adherence (urine samples) for the first time, we were able to show that results are not biased by altered medication and by its adherence. |
Acknowledgements
The authors thank the study nurses (Clinical Research Unit) and the assistant personnel of the angiography lab for invaluable support with patient management.
Conflict of interest statement
A. Schmid has received speaker’s fees from Medtronic. R. Schmieder has received travel support, speaker’s and consultancy fees and institutional grants from Medtronic. The other authors have no conflicts of interest to declare.

