Abstract
Endovascular renal denervation techniques have been clinically adopted for the treatment of resistant arterial hypertension with great success. Despite the favourable early results achieved with this technology, a clear understanding of the pathophysiology underlying this novel treatment is lacking. In addition, non-responsiveness to renal denervation remains a nidus for treatment failure in distinct patients.
In search of meaningful surrogate parameters relating to treatment responsiveness, the current article reviews the existing knowledge on renal nerve anatomy, changes occurring after denervation and procedural parameters collected during denervation.
From preclinical experience, the most reliable morphological parameter reflecting successful renal denervation is the presence of axonal degeneration. Most procedural and clinical parameters need extended investigation before adopting them as potential surrogate parameters for successful renal denervation.
As a consequence, there is an imperative need for dedicated research revealing the pathophysiology of renal denervation procedures. In this regard, close co-operation of engineers, researchers and clinicians is warranted to turn renal denervation into a milestone treatment of arterial hypertension.
Introduction
While renal denervation has been widely accepted as a promising option for patients suffering resistant arterial hypertension, a substantial proportion of patients remains unresponsive1,2. While the reason for the failure of renal denervation in these patients remains unclear, concern has been raised as to the absence of appropriate surrogate parameters that may help to distinguish responders from non-responders.
In an attempt to characterise a successful renal denervation procedure, the current article focuses on the topography of residing renal nerves and the changes occurring after denervation procedures. Furthermore, it provides basic insights into the different technologies and discusses the different parameters collected during the procedure that may facilitate a better prediction of treatment responsiveness.
Lessons learned from preclinical studies: anatomy of renal nerves
Pathologic studies of human renal artery specimens and preclinical models using sheep and pigs have demonstrated that renal sympathetic nerves are distributed around the circumference of the renal artery within the adventitia and periadventitial soft tissue. Neural recognition markers applied to renal sympathetic nerves have further elucidated nerve composition, revealing a mixture of efferent and afferent nerve fibres3. The proximity of these nerves to the renal artery renders them susceptible to a variety of percutaneous catheter-based treatment options specifically aimed at ablation of renal nerves for the treatment of resistant hypertension.
Experience with radiofrequency ablation
Since Huang et al first introduced radiofrequency (RF) catheter ablation4, it has become one of the most useful and widely accepted therapies in the field of electrophysiology. Modifications in RF delivery and improvements in electrode design have resulted in a significant expansion of its indication.
PHYSICAL ASPECTS
RF energy is a form of alternating electrical current that produces a lesion by two mechanisms: 1) direct resistive heating of the tissue in contact with the catheter tip, and 2) thermal conduction or passive heat transfer to deeper tissue layers. While resistive heating in regions close to the RF current source is rapid, passive heat transfer to deeper layers is a slower process5. The heat transfer continues even after discontinuation of RF current delivery, which may result in lesion expansion even after RF current cessation.
The RF electrical current can be delivered in a bipolar mode (between two closely opposed electrodes) or, much more frequently, in a unipolar mode, with completion of the circuit via an indifferent electrode placed on the skin. RF current is delivered to specific regions through transvenous electrode catheters with a catheter tip of 4 to 10 mm (Figure 1).
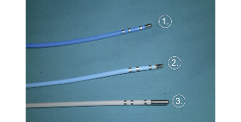
Figure 1. Catheters most frequently used for RF ablation. 1) cooled-tip RF catheter; 2) standard 4 mm RF catheter; 3) standard 8 mm RF catheter
FACTORS THAT INFLUENCE LESION FORMATION
The effects of RF energy on vascular tissue depend on multiple factors such as temperature, duration of current application, power, electrode size, quality of electrode-tissue contact and histological characteristics of the tissue including blood supply and proximity to major blood vessels that determine the degree of heat dissipation.
TEMPERATURE
Tissue temperatures of 50°C or higher are necessary for irreversible damage6. With increasing distance of the electrode, the temperature of the tissue decreases in a hyperbolic mode. In general, a target tissue temperature of 50 to 70°C is used for conventional RF ablation7.
At temperatures above 100°C, boiling may occur in the tissue surrounding the electrode. This results in denaturation of plasma proteins, while coagulation may be activated resulting in charring or coagulum formation (Figure 2)8. An important sign indicating charring or coagulum formation is a sudden rise of impedance instead of a gradual decrease that accompanies successful RF energy delivery. Of note, coagulum formation increases the risk of thromboembolism.
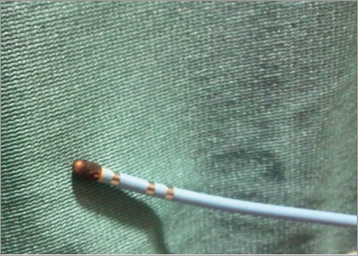
Figure 2. Charring with 8 mm tip electrode catheters.
DURATION OF APPLICATION
In myocardial ablation procedures, the duration of energy delivery should be at least 35 to 45 seconds to achieve steady state temperature9. While the optimal duration of radiofrequency application in renal denervation procedures remains a topic of debate, a minimum duration of 30-60 seconds is needed with most available devices to obtain effective ablation.
POWER
The extent of successfully ablated tissue is proportional to the applied power of the radiofrequency source. Generally, energy delivery is regulated by temperature control that is determined by setting a default target temperature with adjustment of energy to maintain this goal. One of the important drawbacks of this energy delivery is the risk of charring.
ELECTRODE SIZE
Electrode size influences the volume of the ablation lesion and probably the clinical efficacy of RF ablation as well. Generally speaking, larger electrodes result in larger lesions which may provide greater instantaneous efficacy10,11. However, for renal denervation procedures, the relationship between lesion size and treatment efficacy remains to be established.
QUALITY OF ELECTRODE/TISSUE CONTACT
Tissue contact of the radiofrequency electrode may be assessed by measuring baseline impedance. As tissue is heated, there is a temperature-dependent drop in electrical impedance. In the study of Ko et al, a significant positive correlation between pre-ablation impedance and heating efficacy was demonstrated. A similar association was observed between maximum drop in impedance during energy delivery and heating efficacy12.
Temperature monitoring also provides useful information about the quality of electrode/tissue contact. It has been reported that temperature rise is greater and faster with properly engaged electrodes13.
ANATOMICAL SETTINGS AND CHARACTERISTICS OF THE TISSUE
Blood supply and proximity to major blood vessels determine the degree of heat dissipation and represent another factor that may influence optimal lesion formation. Convective heat dissipation via blood flow acts both at the level of tissue and at the electrode tip. At the tissue level, convective heat dissipation removes heat from the tissue limiting the penetration depth of radiofrequency current14.
COOLED-TIP RF ABLATION
Increased energy delivery to tissue can be achieved by active cooling. The most used active cooling system infuses cold saline which exits the catheter through small perfusion holes located adjacent to the catheter tip (open irrigated cooling) (Figure 1). The success of cooled-tip catheters in ablation procedures relates directly to the ability of delivering higher power to the ablation electrode in the absence of excess heating of the tip. In this regard, coagulation and charring are substantially reduced. Experimental studies have shown that active cooling results in higher tissue temperature resulting in larger and deeper lesions compared to conventional RF ablation (Figure 3)15-17.
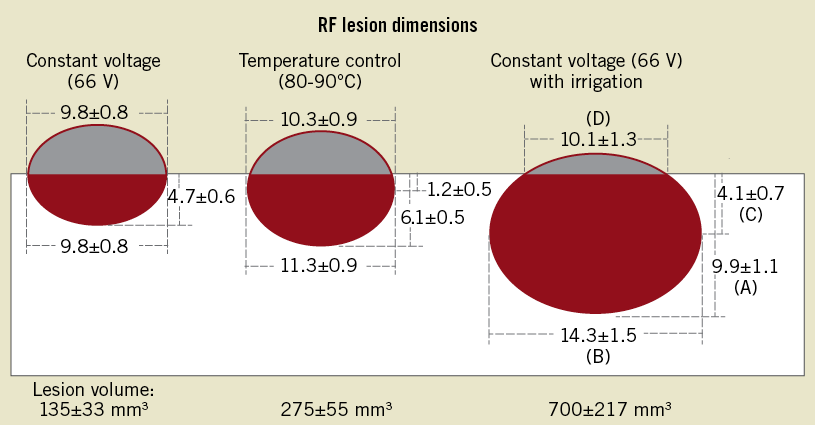
Figure 3. Diagram of lesion dimensions for three groups studied. A indicates maximal lesion depth, B maximal lesion diameter, C depth at maximal lesion diameter, and D lesion surface diameter. Lesion volume was calculated by use of the formula for an oblate ellipsoid by subtracting the volume of the “missing cap”. From Nakagawa H et al17.
A major drawback of cooled-tip ablation is that temperature guidance of energy delivery may be offset due to the inherent limitation of temperature misinterpretation owing to continuously cooled saline infusion.
As a result of higher tissue temperatures, sudden boiling with steam production can occur. However, most contemporary cooled-tip catheters employ temperature-feedback power control, maintaining a lower reference temperature (40-50°C) compared to conventional catheters. The slow impedance drop of cooled-tip ablation catheters may also be landmark, as suggested by clinical experience18.
Preclinical experience using radiofrequency ablation of renal nerves is based on two dedicated studies using the Medtronic Symplicity™ device (Medtronic, Minneapolis, MN, USA) in healthy porcine renal arteries19,20. In the study by Steigerwald et al, the acute and subacute vascular and neuronal findings after renal denervation were characterised by angiography, optical coherence tomography (OCT) and histopathology. Blood chemistry parameters, most importantly renal function and blood glucose levels, remained constant during the procedure and at follow-up. Acute angiographic data were consistent with findings in patients, where vessel notches and spasm were observed acutely following treatment. At 10 days after denervation procedures, vessel notches were still discernable but were absent in a later preclinical safety study of renal denervation using the Medtronic Symplicity™ system at six months20 (Table 1, Figure 4). Post-procedural OCT analysis was helpful in detecting luminal thrombus formation and cellular depletion of the medial tissue at the treatment site. This observation was validated by histopathology where thrombus formation was attributed to loss of the endothelial layer. The endothelial layer was restored after 10 days, while the media displayed focal fibrotic compression. Importantly, the adventitial tissue showed a strong inflammatory response along with neovascularisation. Transmural fibrosis was also seen at six-month follow-up in the study by Rippy et al20. However, intimal hyperplasia and adventitial inflammation were not observed in this study. Despite the presence of transmural injury vessel wall integrity was maintained.
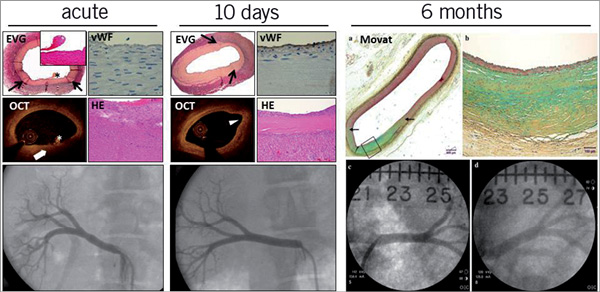
Figure 4. Histopathological and angiographic images of renal arteries following RF treatment using the Medtronic Symplicity™ device. Acutely after treatment the vessel wall shows thermal injury evidenced by oedema and cell depletion. There is absence of endothelium at the treatment site and minimal thrombus formation is evident (*). Vessel notches are obvious at angiography. Ten days later, the media shows fibrosis and compression. There is minimal intimal thickening and re-endothelialisation is complete. Transmural fibrosis is evident at 6 months. Black arrows depict treatment site margins. Six months data were derived from Rippy et al, 201120. EVG: Elastica van Gieson staining; HE: haematoxylin and eosin staining; OCT: optical coherence tomography; vWF: von Willebrand factor staining for presence of endothelial cells
Radiofrequency energy, applied at mean impedance values of 202 Ω, directly affected nerve fascicles19. These showed moderate to severe degenerative morphological changes as early as four to six hours after treatment (Figure 5), which was accompanied by loss of positive immunostaining for the ubiquitous axonal marker neurofilament protein19. These data demonstrate that RF ablation effectively targets nerve fascicles with sustained degeneration in the long term.
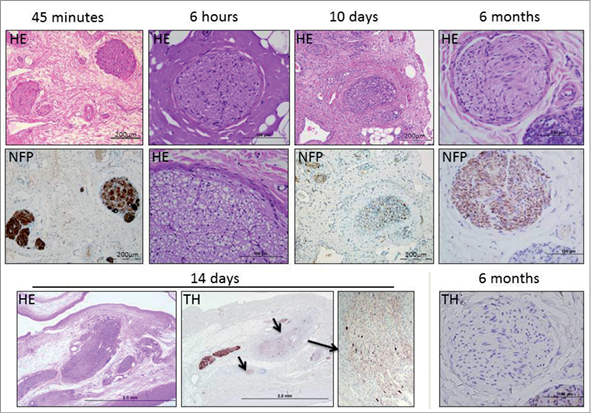
Figure 5. Presentation of nerve fascicles targeted by RF energy at various time points post treatment. Nerve fascicle necrosis and vacuolisation can be found as early as six hours after treatment using the Medtronic Symplicity™ device. Perineural delamination and fibrosis as well as moderate to severe inflammation are evident at 10 days. At this time point, nerve fascicles displayed less intense staining for neurofilament protein (NFP) and tyrosin hydroxylase (TH, arrows). At 6 months, NFP staining was observed while TH staining was absent. Six-hour, 14-day and 6-month data derived from CVPath Institute Inc., unpublished data. HE: haematoxylin and eosin
The clinical consequences arising from preclinical experience mainly relate to the notion that anticoagulation seems to be necessary during and following renal denervation procedures. Despite the well-established safety profile of radiofrequency ablation, vascular injury including endothelial damage is present and may cause thrombotic complications without appropriate anticoagulation. In the aftermath of renal denervation, antiplatelet treatment is strongly recommended for several weeks until vascular healing is complete.
OCT has proven to be of value to detect focal thrombus formation after renal denervation19 and may be of clinical relevance to monitor vascular complications.
Experience with cryoablation
Cryotherapy ablation has been applied for the treatment of cardiac arrhythmias since the late 1990s; however, refinement of the technology and advances in catheter design have clearly expanded the spectrum of cryotherapy ablation procedures.
Generally speaking, the application of cryotherapy to a certain tissue results in the formation of a hemispherical block of frozen tissue. If tissue cooling reaches the temperature of –40°C or beyond, intracytoplasmic ice formation occurs which causes cell death.
The dimension of cryothermal lesions depends on several factors such as temperature, size of the cryothermal probe, application duration of cryothermal energy and number of freeze/thaw cycles. For any given duration of cryothermal exposure, lower temperatures generate larger lesions. On the other hand, at a given temperature lesion size reaches a plateau after five minutes. Furthermore, repetitive freeze/thaw cycles increase the thermal conductivity of the tissue and may explain progressive damage with the increase in the freeze/thaw cycles21. As with RF ablation, larger cryotherapy probes result in deeper cryothermal lesions.
Advantages of cryotherapy ablation include reversibility of cryothermal lesions (at a temperature of –30°C), decreased risk of thrombus formation, increased stability and decreased risk of injury of vascular structures22. However, a higher recurrence rate after successful index ablation has been observed compared to RF ablation22.
Preclinical data for cryotherapy ablation of renal arteries comes from a single experimental study, in which a standard 6 mm tip cryotherapy catheter was used for renal denervation in a sheep model23. By angiography, vascular segments showed an irregular morphology after cryotherapy application, which was absent after three months of follow-up. Lesions displayed transmural coagulation necrosis; however, there was no sign of thrombus formation, with the endothelial layer remaining intact. Nerve fascicles were affected to a similar extent compared to what has been reported with RF ablation, indicating that cryotherapy ablation is similarly effective.
Experience with ultrasound ablation
Ultrasound (US) is another form of energy that can cause thermally mediated tissue damage. Sound waves with a frequency greater than 20 kHz are generally considered to be within the range of ultrasound. When applied to an absorbing medium, US energy is continuously absorbed and converted to heat within the medium.
High intensity focused ultrasound (HIFU) has been shown to be a safe and acceptably effective therapy for surgical ablation of atrial fibrillation24 despite the fact that substantial complications may occur with this technology.
The pathologic effects of selective renal denervation using ultrasound energy consisting of high frequency sound waves (PARADISE™; ReCor Medical, Ronkonkoma, NY, USA) have recently been evaluated. In the acute phase, nerve fascicles demonstrated necrosis and periadventitial inflammation. Over time, histologic features included perineural fibrosis and epiperineural inflammation. Expression of selective neuronal markers (tyrosine hydroxylase and neurofilament protein) correlated with the extent of neural injury25. Furthermore, the early preclinical experience provides evidence that vascular injury is minimal, despite the fact that circumferential residing nerve fascicles are effectively targeted, showing progressive degeneration indicated by necrosis, vacuolisation and ultimately perineural fibrosis (CVPath Institute Inc., unpublished data).
Parameters collected during the procedure
CLINICAL PARAMETERS
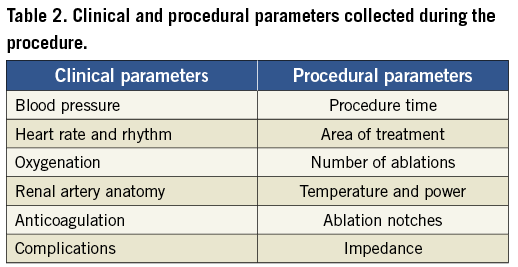
With the first application of radiofrequency energy for renal denervation it became clear that this form of treatment caused substantial pain to the patient. As afferent sympathetic as well as sensory type Aδ and C fibres mediate pain via the dorsal root ganglia to the central nervous system, targeted coagulation of nerve fibres in the adventitia of renal arteries cannot be limited to efferent sympathetic nerves but rather affects the afferent nerve system as well, resulting in substantial pain. Consequently, analgesia and sedation are mandatory attendants of renal denervation procedures. Vital parameters should be thoroughly monitored during the procedure to prevent iatrogenic complications (Table 2).
PROCEDURAL PARAMETERS
As most of the procedural parameters will depend on the technology applied (i.e., radiofrequency vs. cryoablation, ultrasound ablation), some more general parameters applicable to all procedures should be carefully monitored prior to, during and after the procedure. Prior to placing the treatment catheter in the renal artery, a baseline renal angiogram should be performed to assess vessel morphology, presence of atherosclerosis, fibromuscular dysplasia or previously implanted renal stents. In addition, the region and duration of treatment, number of ablations per treatment site and post-procedural abnormalities should be rigorously captured. Potential vascular complications of renal denervation procedures include acute intimal dissections, thrombotic complications, spasm, perforations and fistula as well access site bleedings (Table 2).
The ideal renal ablation device
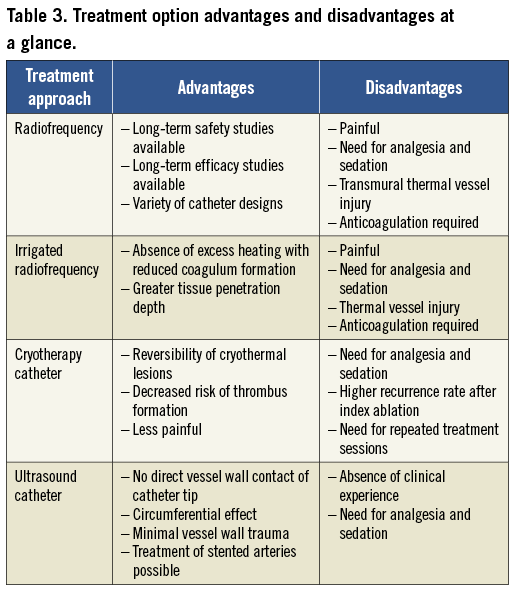
Several key factors may be considered to optimise renal ablation procedures (Table 3).
SELECTION OF ENERGY SOURCE
To date, the largest clinical experience has been gained with RF ablation, which has been applied in electrophysiological procedures for more than 25 years. In keeping with the needs of this innovative technology, radiofrequency ablation offers a well-balanced profile of treatment efficacy and procedural safety. In the randomised Symplicity II study, sustained reduction of hypertension was achieved in the absence of major vascular complications. Therefore, radiofrequency ablation may prevail as the standard of therapeutic options in the invasive treatment of arterial hypertension.
Other energy sources such as cryotherapy or ultrasound ablation hold great potential as future tools in renal denervation procedures; however, clinical data pertaining to both efficacy and safety are lacking to date26.
CATHETER DESIGN
Improvements in catheter design with enhanced stability, modification of RF delivery and electrode design have clearly helped to improve clinical results in the broad spectrum of electrophysiology. In a similar vein, initial experience with the Medtronic Symplicity™ renal denervation device has launched refinement and further improvement of catheter technology for renal denervation procedures.
This has been exemplified in the introduction of cooled-tip catheters, as conventional RF ablation catheters share the limitation of overheating which may increase the risk of endothelial injury and thrombus formation.
More recently, balloon technology and “single shot” devices have expanded the portfolio of renal denervation equipment. For renal denervation procedures, maximum efficacy might be achieved with complete coagulation of adventitial nerves, which has been a limitation of contemporary devices19.
As most nerves are randomly distributed along the circumference of the renal artery and tend to increase from proximal to distal segments27, multielectrode catheters employing balloon technology or different scaffolds may provide further improvement in clinical efficacy.
Summary
Renal denervation, a novel interventional procedure, holds great potential for the treatment of resistant arterial hypertension. Both preclinical and clinical studies have proven the concept of radiofrequency current application to renal arteries in targeting afferent and efferent sympathetic nerves relevant for blood pressure regulation. Unlike other interventional procedures, the initial success with this technology has unleashed a wide range of complementary developments that may facilitate improvement in treatment efficacy in the short to medium term. However, long-term data of clinical success with reductions in cardiovascular mortality are lacking to date.
One of the most intriguing issues related to renal denervation is that the impressive clinical success was not preceded by experimental studies unveiling the pathophysiological sequences occurring after renal denervation. Alongside the presence of non-responding individuals after the first renal denervation procedure, great debate about appropriate surrogate parameters has emerged among the experts in this field. In this regard, the current article aimed to discuss potential surrogates of effective renal denervation on a morphological, technological and clinical level. On a morphological level, the most promising surrogate of effective denervation seemed to be the verification of axonal degeneration as evidenced by the absence of important neuronal markers such as neurofilament protein19. On a technical level, temperature and impedance monitoring was most useful in predicting appropriate catheter tissue contact. With the introduction of cooled-tip catheters, however, the appreciation of temperature monitoring may alter substantially, which likely distracts from adopting it as a clinically relevant surrogate parameter. Clearly, future studies are needed to confirm this hypothesis and to gain more insights into the clinical usefulness of such surrogates. On a clinical level, the presence of pain is a likely predictor of effective distortion of afferent nerves as they provide feedback to the central nervous system. As pain remains an unacceptable attendant of renal denervation procedures, different parameters are needed to distinguish successful from unsuccessful renal denervation procedures.
In the long term, the combined effort of engineers, researchers and clinicians will be necessary to facilitate the understanding of treatment non-responsiveness relative to renal denervation procedures. Only then may renal denervation become a milestone in the treatment of arterial hypertension.
Conflict of interest statement
The authors have no conflicts of interest to declare.

