As the field of interventional cardiovascular medicine has in recent years rapidly spread beyond coronary intervention into the structural heart and endovascular arenas, only a handful of therapeutics have garnered as much interest as renal denervation (RDN) for the treatment of hypertension (HTN). In part, this is a result of the remarkable demonstrations of clinical efficacy of RDN when applied to patients with resistant HTN – findings that have been consistent across a range of devices and patient subgroups1,2. These efficacy data, observed in conjunction with very low rates of adverse safety events in the currently existing small to moderately sized clinical trials of RDN therapies, have set the stage for what some would consider an impending “explosion” of use of RDN for the treatment of HTN and beyond.
In anticipation of the significant interest in more widespread utilisation of RDN therapies, there have been several professional society-based efforts both to establish and to describe consensus best practices for this therapy1-3. In addition to establishing practice standards, these consensus documents can help the practising clinician to understand the pathophysiologic basis for RDN and to assess objectively the strengths and limitations of the current evidence base studying the clinical use of RDN therapies. In this regard, the document by Tsioufis et al, in the current issue of EuroIntervention, builds upon prior consensus efforts by focusing in greater detail specifically upon the procedural aspects of RDN, and should serve as a welcome reference for proceduralists wishing to perform RDN in their practice.
Particular attention is paid to several aspects of the procedure that may be less readily familiar to endovascular interventionalists with only limited knowledge of renal artery interventions, including: variations of renal artery anatomy, the use of non-invasive imaging to assess renal arteries, the location and anatomy of renal sympathetic nerves and, importantly, several technical procedural details including the management of periprocedural medications during RDN, such as sedation and antithrombotic therapy. Although there has been rapid differentiation and expansion of technologies to perform RDN, the technology-by-technology summaries of the various current devices within the document will also serve as an opportune refresher for those who have not followed this growing field closely.
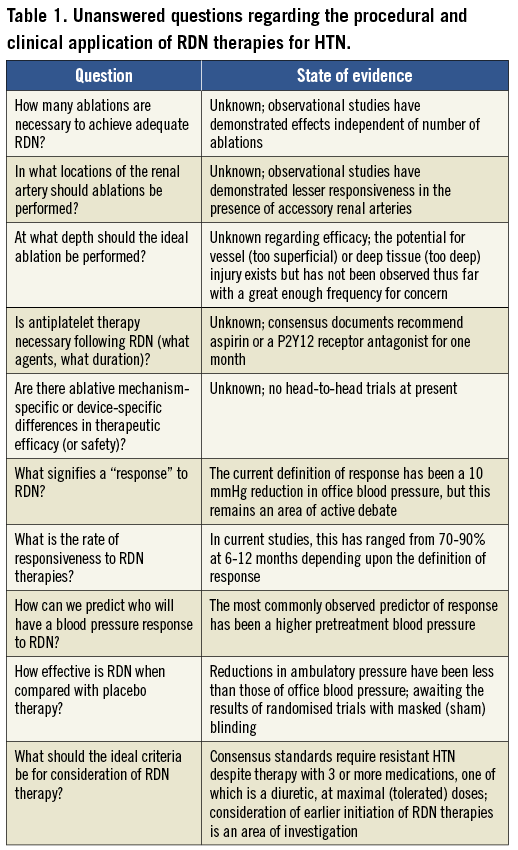
Ultimately, the most appropriate clinical use of these various RDN devices will depend upon each therapy providing strong clinical evidence of efficacy with an acceptable safety profile. It is remarkable that, despite sky-high expectations for a technology that many consider well beyond its infancy in the European Union and Australia, RDN therapies are not even approved outside of clinical trials in the United States, Japan, China, and a host of other countries. Moreover, several important and fundamental questions regarding RDN therapies at present remain unanswered and are under active investigation (Table 1). One of the most intriguing aspects of RDN therapy has been the recent concern that the effectiveness of RDN therapies on blood pressure has been grossly overestimated due to the potential for systematic bias in single-arm and non-placebo-controlled studies of RDN therapies4. Although observational studies of RDN have demonstrated significant reductions in blood pressure of patients with non-pseudoresistant HTN as ascertained by both office and ambulatory monitoring5, the results of the pivotal SYMPLICITY HTN-3 trial (as well as other future and ongoing randomised trials) are eagerly anticipated in order to assess the true efficacy of RDN when applied in a sham-controlled design employing masked blinding and endpoint ascertainment6.
As we wait for the results of these trials, the excitement and interest in RDN therapies is only growing. At the last count, there were >100 registered studies on www.clinicaltrials.gov studying RDN with an expanding range of devices and indications for therapies (including those beyond HTN). However, in addition to becoming familiar with the technologies and practical application of the RDN procedure itself, those who practise interventional cardiovascular medicine would additionally be well served by becoming more familiar with the preprocedural work-up and evaluation of patients with resistant HTN. Although the scope of the present document by Tsioufis et al is by design primarily limited to procedural aspects of RDN, this document should be viewed in conjunction with other existing consensus documents that are able to devote more attention to the appropriate evaluation and management of resistant HTN as a whole1,2.
In order to understand which patients will benefit most from RDN, we must familiarise ourselves better with the fundamentals and pathophysiology of the HTN disease process itself. Increasingly, we as proceduralists are asked to extend our knowledge beyond technical skills alone and to participate actively in shared decision-making with our patients and with other treating physicians (such as hypertension specialists). If we are unable to transfer our expertise outside the procedural suite or catheterisation laboratory and into the office-based treatment of our patients with HTN, we potentially stand to compromise the overall clinical benefit of a highly effective therapy by misapplication of RDN to patients who may benefit less from the procedure. As with any other therapeutic intervention (particularly an invasive one), the most effective application of RDN therapy will be observed when it is applied to the most appropriate candidates for the therapy: those with the most to gain, and the least to lose.
Conflict of interest statement
The authors have no personal conflicts of interest to declare. Institutional research grants have been provided to Columbia University by Medtronic, Boston Scientific, and St. Jude Medical.

