Abstract
Aims: To evaluate the safety and efficacy of the balloon-based bipolar Vessix Renal Denervation System in treating patients with resistant hypertension.
Methods and results: In this prospective, multicentre, single-arm study, 146 patients (age 58.6±10.5 years; 61% men) with office systolic blood pressure (BP) ≥160 mmHg despite ≥3 antihypertensive medications at maximally tolerated doses were treated with the Vessix System. Efficacy endpoints were reductions in office and 24-hour ambulatory systolic and diastolic BPs at six months. Acute and long-term safety, with a focus on the renal artery and estimated glomerular filtration rate (eGFR), were assessed. Baseline office and ambulatory BPs were 182.4±18.4/100.2±14.0 mmHg and 153.0±15.1/87.5±13.2 mmHg, respectively. No acute renal artery injury requiring intervention or serious periprocedural cardiovascular events occurred. At six months, office BP was reduced by 24.7±22.1/10.3±12.7 mmHg (p<0.0001) and ambulatory BP was reduced by 8.4±14.4/5.9±9.1 mmHg (N=69; p<0.0001). Twenty-six patients (18%) achieved an office systolic BP <140 mmHg. One patient had renal artery stenosis which required stenting. Mean eGFR remained stable.
Conclusions: Renal artery denervation with the Vessix System reduced both office and ambulatory BP at six months in patients with resistant hypertension. Renal artery safety and renal function results are favourable.
Introduction
The relationships between elevated BP and increased cardiovascular and renal-related morbidity and mortality risks are well known1,2. Conversely, lowering BP in patients with hypertension yields benefits such as decreased incidence of stroke, major cardiovascular events, end-stage renal disease, and death1,3,4.
Renal denervation provides a novel adjunctive option for treating patients with hypertension despite lifestyle modifications and multiple antihypertensive medications5,6. Clinical studies of percutaneous renal denervation with radiofrequency energy have shown significant and sustained BP reductions for patients with resistant hypertension following treatment with either single-electrode7-10 or multielectrode11 monopolar systems. The Vessix™ Renal Denervation System (Boston Scientific, Marlborough, MA, USA), by contrast, is a balloon-based bipolar multielectrode system.
We report here the six-month results of the REDUCE-HTN study, which was designed to evaluate safety and efficacy of renal denervation with the Vessix System for the treatment of resistant hypertension.
Methods
STUDY DESIGN
The REDUCE-HTN clinical study is an international, prospective, non-randomised, single-arm study. This report describes the study results obtained up to six months (i.e., the primary endpoint) for patients enrolled in the initial first-in-man cohort and those enrolled under an amended post-market protocol. The REDUCE-HTN study is registered at ClinicalTrials.gov (NCT01541865).
The study was conducted in accordance with ethical principles which have their origins in the Declaration of Helsinki. Institutional committees on research or appropriate ethics committees at study sites approved the study protocol. Patients were required to provide written informed consent prior to receiving any study-specific tests or procedures.
PATIENTS
Inclusion and exclusion criteria for the first-in-man and post-market protocols are summarised in Table 1. Eligible patients had office-based systolic BP ≥160 mmHg and were on a stable medication regimen with at least three antihypertensive drugs (including a diuretic, unless intolerant) at maximally tolerated doses.
BP, renal function, and renal artery anatomy were assessed at the screening visit. Seated office BP measurements2 were taken with a validated electronic device (Omron model HEM-705 CP; Omron Healthcare, Lake Forest, IL, USA). Eligible patients had an estimated glomerular filtration rate (eGFR) ≥45 ml/min per 1.73 m2. Patients underwent renal duplex ultrasound, computed tomography angiography, or magnetic resonance angiography to screen for anatomic abnormalities. Antihypertensive medication regimen stability over the previous two weeks was confirmed by reviewing the patients’ medical records and patient-reported compliance.
VESSIX RENAL DENERVATION SYSTEM
The Vessix Renal Denervation System comprises the Vessix catheter and generator. The catheter is an over-the-wire low-pressure (3 atm) balloon catheter designed to transmit radiofrequency energy via multiple bipolar electrodes mounted on its surface in a helical pattern. The catheters used in this study were compatible with 0.36 mm (0.014 inch) and 0.46 mm (0.018 inch) guidewires and 8 Fr guide sheaths; the balloons had 4, 5, 6, or 7 mm diameters and four to eight bipolar electrodes (depending on their size).
The Vessix balloon enables electrode apposition to the artery wall while eliminating variable cooling from blood flow. The generator delivers radiofrequency energy simultaneously to all apposed electrodes and adjusts to maintain a 68°C temperature, while thermistors on the balloon surface monitor temperature throughout the 30-second treatment period. The temporary no-flow environment, bipolar electrodes, and temperature control enable a therapeutic temperature to be reached with <1 W12.
RENAL DENERVATION PROCEDURE
Patients who met general and anatomic criteria underwent standard angiography to confirm renal artery anatomy suitability immediately prior to the denervation procedure. Anxiolytic and analgesic medications were administered and systemic anticoagulation (e.g., activated clotting time ≥200 seconds) was attained. The Vessix catheter was delivered to the renal artery via femoral access and inflated using standard angioplasty techniques. After acceptable apposition was confirmed, the generator was activated to deliver radiofrequency energy. The balloon could be deflated, moved proximally, and re-inflated in order to treat along the full artery length. No more than two treatments per artery were recommended. The balloon was then positioned in the opposite renal artery and the radiofrequency treatment procedure was repeated. Manual compression or commercialised closure devices were used to achieve haemostasis at the puncture site.
FOLLOW-UP PROCEDURES
Patients were instructed to remain compliant with their baseline antihypertensive medication regimen throughout the study unless changes were clinically indicated. Seated BP measurements2 were repeated during office visits at one, three, and six months following the renal denervation procedure. Ambulatory BP over 24 hours was monitored with Spacelabs ABP monitors (Spacelabs Healthcare, Snoqualmie, WA, USA) and validated by a core laboratory (Biomedical Systems Corporation, St. Louis, MO, USA) at baseline and six months. A 70% success rate (e.g., excluding movement artefacts) from hourly recordings over 24 hours was required. Monitoring results that did not meet the required validation criteria were excluded from analysis.
Renal function was monitored with eGFR and creatinine levels. A renal artery duplex ultrasound was required at six months and reviewed by an independent core laboratory (VasCore, Boston, MA, USA). Angiographic images were also evaluated by a core laboratory (SynvaCor, Springfield, IL, USA).
EFFICACY AND SAFETY ENDPOINT DEFINITIONS
Efficacy was assessed as the magnitude of the changes in office-based and 24-hour ambulatory BPs from baseline to six months following treatment with the Vessix System.
The primary first-in-man study objective was to assess acute safety, defined as freedom from each of five periprocedural events: renal artery dissection/perforation that required stenting or surgery, renal artery infarction/embolus, cerebrovascular accident, myocardial infarction, and sudden cardiac death. These acute events continued to be monitored for the expanded cohort.
Long-term safety endpoint events were: chronic symptomatic orthostatic hypotension, hypertensive emergency necessitating hospital admission (unrelated to antihypertensive medication non-compliance), eGFR reduction >25%, angiographically documented renal stenosis requiring an intervention, and flow-limiting stenosis (≥60%) in the renal artery.
A data safety monitoring board adjudicated all adverse events for seriousness and relatedness to the procedure and device.
STATISTICAL METHODS
All treated patients were included in the analyses. The study sample size was not powered for efficacy or safety endpoints. Descriptive statistics are presented and confidence intervals (95%) were constructed for BP results. A paired t-test was used to assess the BP change from baseline to six months; the data normality assumption was verified with the Shapiro-Wilk test. A p-value <0.05 was considered statistically significant.
Post hoc office BP reduction comparisons in patient subgroups were made with two-sample t-tests. Logistic regression was used to analyse the relationships between baseline/treatment characteristics and a six-month office systolic BP reduction >10 mmHg or ambulatory systolic BP reduction >5 mmHg. All analyses were conducted with SAS version 9.2 or later (SAS Institute Inc., Cary, NC, USA).
Results
PATIENT CHARACTERISTICS
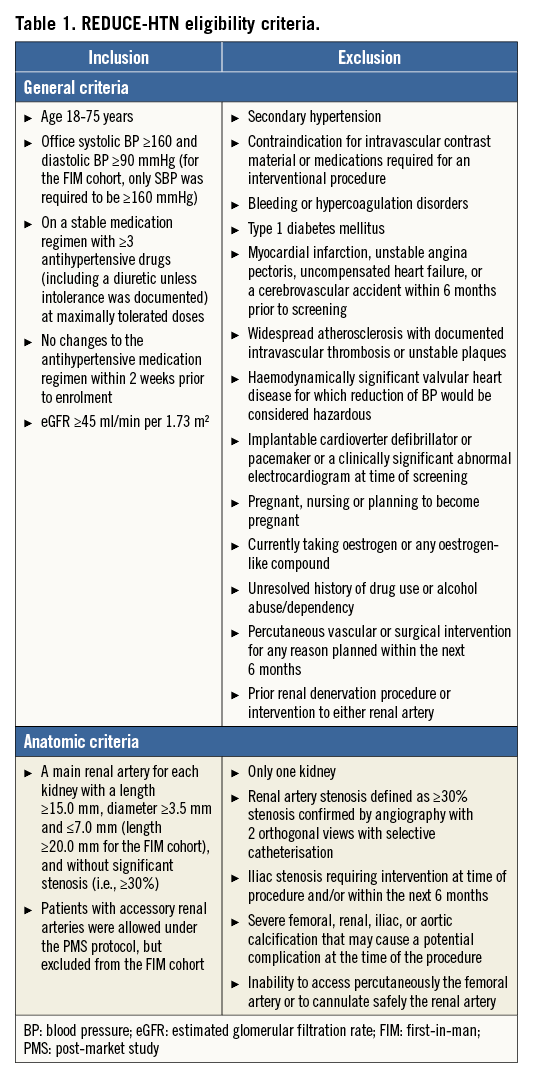
A total of 146 patients were treated with the Vessix System at 23 sites in Europe, Australia, and New Zealand from February 2012 to April 2013 (Figure 1, Online Appendix 1). Baseline patient characteristics are shown in Table 2. The mean (±SD) diameter of treated renal arteries was 5.3±0.7 mm and length was 34.7±8.4 mm. Twenty-four patients enrolled under the post-market protocol had accessory renal arteries treated.
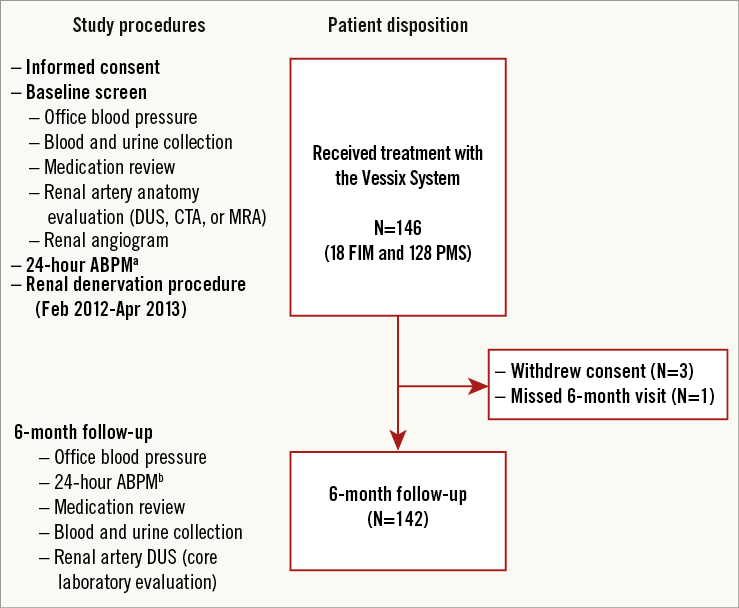
Figure 1. REDUCE-HTN patient flow. ABPM: ambulatory blood pressure monitoring; CTA: computed tomography angiography; DUS: duplex ultrasound; FIM: first-in-man; MRA: magnetic resonance angiography; PMS: post-market study. a103 patients had valid 24-hour ABPM at baseline. b89 patients had valid 24-hour ABPM at 6 months; 69 of these patients also had valid baseline ABPM.
Nine patients who received radiofrequency treatment met exclusion criteria: one had baseline systolic BP of 157 mmHg, two had eGFRs below the eligibility threshold, two were on hormonal therapy, one had a pacemaker, one had renal artery length <15 mm, one had a previous renal denervation and renal artery stenosis >30%, and one had been placed on an antihypertensive drip within two weeks prior to the procedure. None of these patients experienced procedure-related adverse events associated with these baseline conditions during six months of follow-up.
PROCEDURE CHARACTERISTICS
Catheters with 7 mm (35%) or 6 mm (34%) balloon diameters were selected most often; the remainder were 5 mm (22%) and 4 mm (9%). Patients underwent a mean of 3.5±1.1 treatments (i.e., 30-second generator activations) with 21.2±6.4 electrode activations. All patients had at least one complete treatment cycle. To treat the full artery length, investigators administered two treatments in 54% of main renal arteries (158/292) and 41% (11/27) of treated accessory arteries. Treatments required 0.7±0.2 W per electrode to achieve temperature control. Mean procedure time (i.e., first balloon inserted to last balloon removed) was 24.9±15.8 min.
CHANGES TO ANTIHYPERTENSIVE MEDICATION REGIMENS
At six months, the mean number of antihypertensive medications per patient remained stable at 5.2±1.9, and 85.6% remained on the same number of medications as at baseline. Fifteen (10.3%) reduced their antihypertensive regimen by one drug, two patients reduced their regimen by two medications, three reduced their regimen by three medications, and one was on four fewer antihypertensive drugs at the six-month follow-up visit than at baseline; no patient had an increased number of antihypertensive drugs.
Efficacy endpoints
OFFICE BLOOD PRESSURE
Significant office-based BP reductions were observed at all follow-up time points (Figure 2). At six months, systolic/diastolic BP was reduced by 24.7±22.1/10.3±12.7 mmHg. Systolic BP reductions ≥5 mmHg or ≥10 mmHg were observed in 85% (121/142) and 76% (108/142), respectively. Six patients (4%) had a reduction in systolic BP of <5 mmHg, and 15 (11%) had an increase at six months. Systolic BP <140 mmHg was achieved by 18% (26/142) of patients at six months (Figure 3).
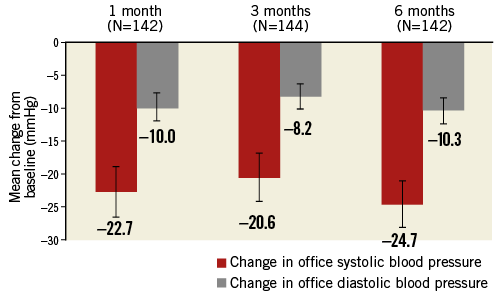
Figure 2. Change in office-based blood pressure (95% confidence intervals). p<0.0001 for each time point vs. baseline.
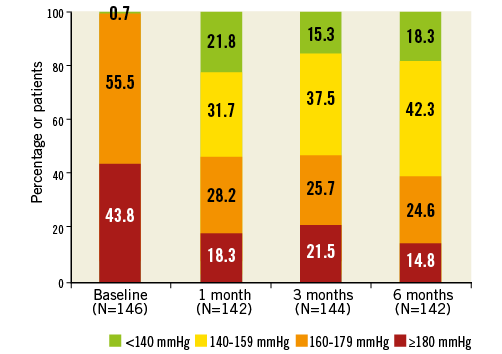
Figure 3. Distribution of office systolic blood pressures at baseline and follow-up up to six months.
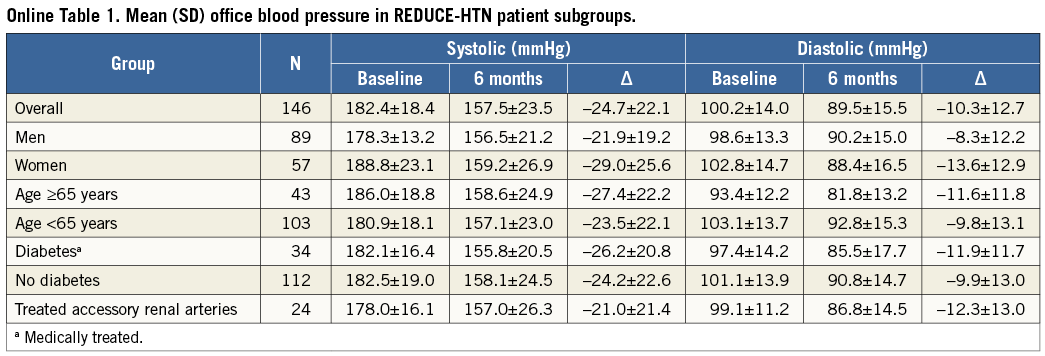
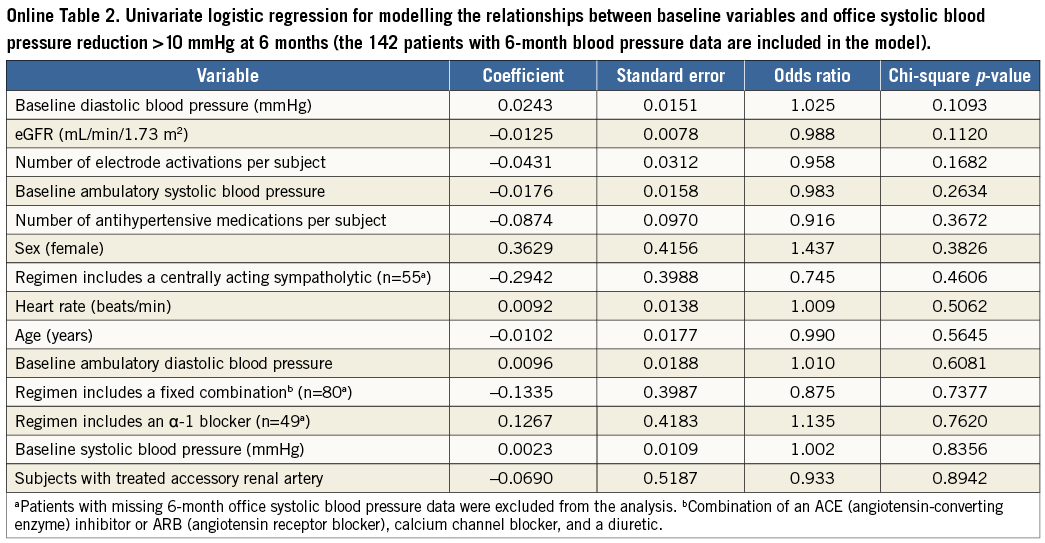
No significant differences in the changes in office systolic BP at six months were observed between subgroups based on age, diabetic status, or sex (p>0.05 for each comparison) (Online Table 1). The mean (±SD) BP reduction observed among patients without any changes in the number of antihypertensive medications was –23.8±21.2/–9.8±12.2 mmHg, and for the 21 patients who decreased their number of medications it was –29.5±26.8/–13.7±15.2 mmHg. No significant relationships were identified between baseline characteristics and systolic BP reduction >10 mmHg at six months in logistic regression analysis (Online Table 2).
24-HOUR AMBULATORY BLOOD PRESSURE
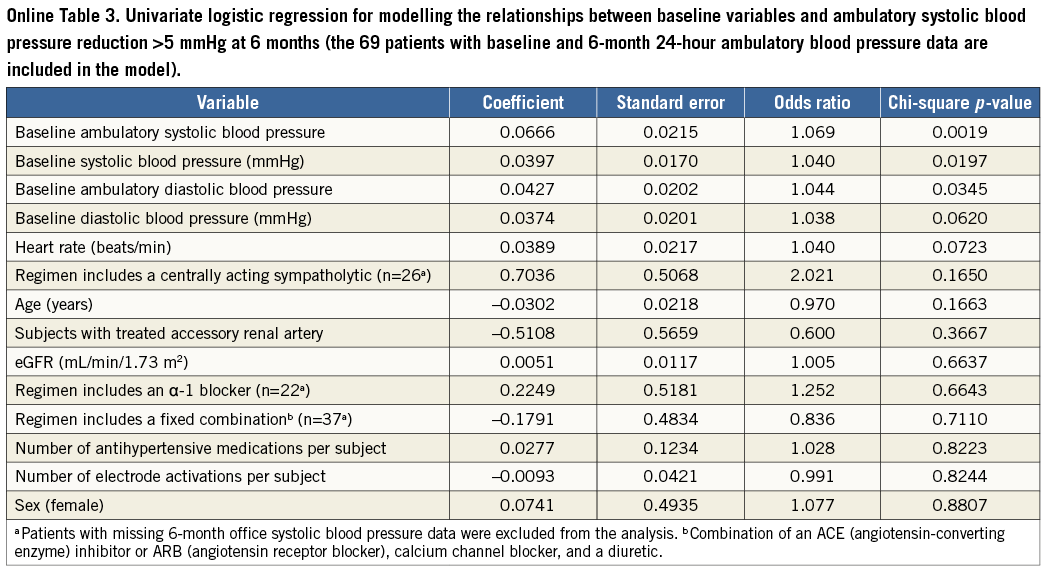
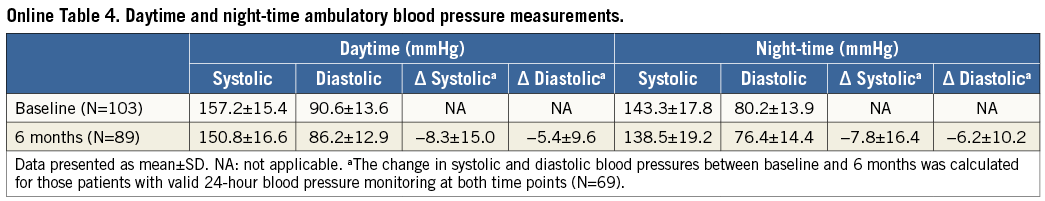
Baseline mean (±SD) 24-hour ambulatory BP was 153.0±15.1/ 87.5±13.2 mmHg (N=103). At six months, mean ambulatory BP decreased to 147.0±16.1/83.1±12.7 mmHg (N=89) and 17% of patients (15/89) had systolic pressure <130 mmHg (vs. 4% [4/103] at baseline). The mean reduction among the 69 individuals with valid 24-hour ambulatory measurements at baseline and six months was 8.4±14.4/5.9±9.1 mmHg (p<0.0001) (Figure 4). In logistic regression analysis, greater baseline systolic BP (office or ambulatory) and greater ambulatory diastolic BP were associated with greater odds of a six-month reduction in ambulatory systolic BP >5 mmHg (Online Table 3). The mean changes in daytime and night-time ambulatory BP were similar (Online Table 4).
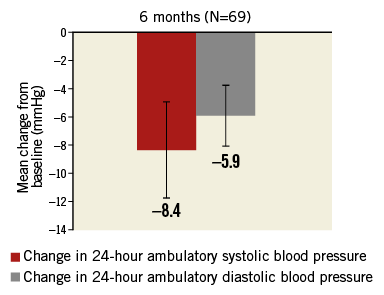
Figure 4. Change in 24-hour ambulatory blood pressure (95% confidence intervals). p<0.0001 for both systolic and diastolic vs. baseline.
Safety objectives
ACUTE SAFETY
No patient had periprocedural renal artery dissection or perforation that required intervention, or renal artery infarction or embolus. One mild procedural vessel dissection which did not require intervention was reported. No cerebrovascular accidents, myocardial infarctions, or sudden cardiac deaths occurred within 30 days. Seven serious adverse events that were determined by the data safety monitoring board to be related to the procedure occurred within the first month post procedure, including two access-site infections, one pseudoaneurysm at the access site, and one femoral artery thrombus. One haematoma, one instance of bilateral flank pain, and one case of vomiting, all of which occurred within one day of the procedure, were classified as serious because hospitalisation was prolonged for observation. All events have resolved.
LONG-TERM SAFETY ENDPOINTS
Up to six months, one patient had a hypertensive emergency necessitating hospital admission and none had symptomatic orthostatic hypotension. Fifteen patients (11%) had an eGFR reduction >25% at six months. Two of these 15 patients presented with acute renal insufficiency which was related to medication use: one occurred approximately three months post procedure and was treated by lowering the diuretic dose, and the other was treated by withholding an ACE inhibitor and statin at approximately six months post procedure. Mean eGFR and serum creatinine remained stable at 82.9±23.7 mL/min per 1.73 m2 (change: −0.9±16.4 mL/min per 1.73 m2; N=138) and 85.2±24.2 µmol/L (change: 2.4±13.4 µmol/L; N=140) at six months, respectively.
Evaluable duplex ultrasounds were obtained from 123 patients at the six-month follow-up visit. Two of these patients had stenosis ≥60% based on core laboratory analysis and underwent subsequent imaging. The first had a 26% left renal artery stenosis at baseline which progressed to 65% in the treated area according to renal angiography conducted approximately seven months post procedure (core laboratory analysis). The second patient had 14% stenosis at baseline, and computed tomography angiography at eight months confirmed moderate left renal artery narrowing. These two patients have not undergone renal angioplasty or stenting and continue to be monitored.
Two additional patients with non-interpretable or abnormal duplex ultrasounds who underwent subsequent imaging studies were found to have renal artery stenoses within six months. One of these patients had 17% stenosis at baseline, and angiography at six months showed 60% stenosis within the treated area of the right renal artery. The patient underwent angioplasty and stenting, and continues to be monitored. This event was considered by the data safety monitoring board to be serious and both procedure- and device-related. The remaining patient had progression of a pre-existing 25% stenosis of the left renal artery to 73% at six months (core laboratory angiogram analysis) which did not need angioplasty or stenting.
Discussion
The REDUCE-HTN study results show that BP was reduced significantly for patients with resistant hypertension following multielectrode balloon-based renal denervation. Significant office-based BP reductions were observed one month post treatment and sustained up to six months, while 24-hour ambulatory monitoring results at six months further support the clinical effectiveness of the Vessix System. BP reductions were similar to results from other uncontrolled and controlled studies six months after treatment13 with various monopolar radiofrequency systems7,9-11,14.
At six months, clinically relevant reductions of at least 5 mmHg15 were observed in 85% of patients (76% had a reduction of ≥10 mmHg): this response rate is within the range reported in previous renal denervation studies (58% to 84%)7,10,11,16-18. Baseline and treatment characteristics were not significantly associated with the office systolic BP response in post hoc analyses, although baseline BP was significantly associated with an ambulatory systolic BP reduction >5 mmHg. These analyses were limited by the subgroup sizes, and additional research is needed to identify or rule out possible predictors of the response to treatment. Achieving systolic BP <140 mmHg reduces the risk for cardiovascular morbidity2, and 18% of patients in the REDUCE-HTN study had BP below this level at the six-month visit. Two previous studies have reported BP control rates of approximately 40% at six months following renal denervation10,11; however, comparisons are limited because baseline BP for patients in REDUCE-HTN was greater on average than in the other studies (182 mmHg vs. 176-178 mmHg), necessitating a greater reduction in order to reach the target level, and because the sample sizes in previous studies were relatively small (<50 subjects assessed vs. 142 subjects in REDUCE-HTN). These findings suggest that radiofrequency treatment with the bipolar balloon-based Vessix System may provide clinically meaningful BP reductions for patients with resistant hypertension.
Like other first-in-man and post-market studies of radiofrequency renal denervation devices, the REDUCE-HTN study was designed as a single-arm, non-blinded, non-randomised study7,9,11,14. The study design is prone to observer bias, and lack of a control arm precludes definitive conclusions regarding efficacy19. However, relevant variables are difficult to control, even with a control arm. The recently published results of the sham-controlled, blinded SYMPLICITY HTN-3 study did not show a significant difference in the BP reduction at six months between patients treated with a single-electrode, monopolar renal denervation device (Symplicity™ Renal Denervation System; Medtronic, Minneapolis, MN, USA) and those in the control arm18. This lack of difference between treatment and control has raised many questions, including whether medication regimens were stable and whether denervation procedures were adequately performed with the single-point device, which may be highly influenced by the operator18,20-22. In contrast to the SYMPLICITY HTN-3 results, Global SYMPLICITY registry23 data showed significant BP reductions for patients who had renal denervation with the Symplicity catheter, including those whose office BP aligned with the HTN-3 criteria21. Additional research is needed to reconcile the results of this and other previous studies with those of the SYMPLICITY HTN-3 trial.
The single-arm design of the REDUCE-HTN study limits our ability to investigate possible confounding effects of antihypertensive medications. Two weeks may be an insufficient duration for pre-treatment changes to the antihypertensive regimen to have observable effects on baseline BP, and patient-reported adherence may inadequately gauge regimen stability. Notwithstanding these limitations, the sample size was relatively large compared with previous uncontrolled studies7,9,11,14, and the inclusion of 24-hour ambulatory BP measurements mitigates observer bias, which is possible with office-based BP measurements19.
Results from the REDUCE-HTN study demonstrate a favourable safety profile for the Vessix System. No acute events indicative of seriously compromised renal artery integrity or cardiovascular complications occurred, and fewer than 6% of patients had serious procedure-related adverse events during six months of follow-up. Mean eGFR remained stable over six months, similar to other studies10,11,16. Of the four patients in this study with renal artery stenosis detected up to six months, only one required angioplasty and stenting. Two were found to have progression of pre-existing stenoses, as has been reported in other studies10,11,14. The requirement for core laboratory evaluation of the six-month duplex ultrasound contributed to the stenosis detection rate, and renal artery stenosis must continue to be examined in larger trials and registries17.
Conclusion
Patients with resistant hypertension were safely treated with a percutaneous balloon-based multielectrode bipolar radiofrequency system and achieved significant, clinically meaningful15, BP reductions over six months of follow-up.
| Impact on daily practice In addition to lifestyle modifications and antihypertensive medications, renal denervation may be a treatment option for some patients with hypertension. This study suggests that denervation with a balloon-based multielectrode bipolar radiofrequency system provides clinically meaningful blood pressure reductions and a favourable safety profile for patients with resistant hypertension. |
Acknowledgements
This work was supported by Vessix Vascular, Laguna Hills, CA, USA, and Boston Scientific, Marlborough, MA, USA. The authors thank Ana Becker for clinical programme management and Elizabeth J. Davis, PhD, for medical writing assistance (Boston Scientific, Maple Grove, MN, USA) and H. Terry Liao, PhD (Boston Scientific, Marlborough, MA, USA) for statistical analysis. Preliminary analyses were presented at VIVA 2013, October 8-11, Las Vegas, NV, USA, at TCT 2013, October 27-November 1, San Francisco, CA, USA, and at CRT 2014, February 22-25, Washington, DC, USA.
Funding
This work was supported by Vessix Vascular, Laguna Hills, CA, USA, and Boston Scientific, Marlborough, MA, USA.
Conflict of interest statement
H. Sievert has received study honoraria, travel expenses and consulting fees from Abbott, Access Closure, AGA, Angiomed, Aptus, Atrium, Avinger, Bard, Boston Scientific, BridgePoint, Cardiac Dimensions, CardioKinetix, CardioMEMS, Coherex, Contego, Covidien, CSI, CVRx, EndoCross, ev3, FlowCardia, Gardia, Gore, Guided Delivery Systems, InSeal Medical, Lumen Biomedical, HLT, Lifetech, Lutonix, Maya Medical, Medtronic, NDC, Occlutech, Osprey, Ostial, PendraCare, pfm Medical, ReCor, ResMed, Rox Medical, SentreHeart, Spectranetics, SquareOne, Svelte Medical Systems, Trireme, Trivascular, Venus Medical, Veryan, and Vessix Vascular; grant support from Cook and St. Jude Medical; and has stock options with Cardiokinetix, Access Closure, Velocimed, Lumen Biomedical, Coherex, SMT. J. Schofer has received speakers’ honoraria from Boston Scientific. U. Hoppe has received grant support and honoraria from Boston Scientific/Vessix Vascular, grant support and consultancy fees from Medtronic, and consultancy fees from St. Jude Medical. I. Meredith has received honoraria and consulting fees from Boston Scientific. M. Azizi has received honoraria for advisory board meetings from Vessix, Boston Scientific, and Cordis, and speakers’ honoraria from Cordis and CVRx. J. Diaz-Cartelle is an employee of Boston Scientific and holds stock in the company. M. Cohen-Mazor is an employee of Boston Scientific and holds stock in the company. The other authors have no conflicts of interest to declare.
Appendix 1. REDUCE-HTN Investigators
Writing Committee: Horst Sievert (CardioVascular Center Frankfurt, Frankfurt, Germany), Joachim Schofer (Universitäres Herz- und Gefäßzentrum, Hamburg, Germany), John Ormiston (Mercy Angiography, Auckland, New Zealand), Uta C. Hoppe (Paracelsus Medical University, Salzburg, Austria), Ian Meredith (MonashHEART, Monash Health, Melbourne, Australia), Darren Walters (The Prince Charles Hospital, Brisbane, Australia), Michel Azizi (Hôpital Européen Georges Pompidou, Paris, France ), Juan Diaz-Cartelle (Boston Scientific, Natick, MA, USA) and Meital Cohen-Mazor (Vessix Vascular, Laguna Hills, CA, USA, and Boston Scientific, Natick, MA, USA).
Clinical Investigators: Uta C. Hoppe (Paracelsus Medical University, Salzburg, Austria), Clemens Steinwender (Allgemeines öffentliches Krankenhaus der Stadt Linz, Linz, Austria), Eric Wyffels (Onze-Lieve-Vrouwziekenhuis, Aalst, Belgium), Ian Meredith (MonashHEART Monash Health, Melbourne, Australia), Horst Sievert (CardioVascular Center Frankfurt, Frankfurt, Germany), Michel Azizi, Marc Sapoval (Hôpital Européen Georges Pompidou, Paris, France), Darren Walters (The Prince Charles Hospital, Brisbane, Australia), Georg Ehret (Cardiology Center, Geneva University Hospitals, Geneva, Switzerland), Mark Webster (Auckland City Hospital, Auckland, New Zealand), Ajay Sinhal (Flinders Medical Centre, Bedford Park, South Australia, Australia), Joost Daemen (Erasmus Medical Center, Rotterdam, The Netherlands), Ralf Langhoff (Vascular Center Berlin, Berlin, Germany), Nicolaus Reifart (Main Tanus Kliniken, Bad Soden, Germany), David Muller (St. Vincent’s, Sydney, Australia), Dierk Scheinert (Zentrum für Gefäßmedizin, Leipzig, Germany), Robbert-Jan de Winter (Academic Medical Center, Amsterdam, The Netherlands), Alexandre Persu (Cliniques Universitaires Saint Luc, Bruxelles, Belgium), Jean Fajadet (Clinique Pasteur, Toulouse, France), Ilka Ott (German Heart Centre, Munich, Germany), Joachim Schofer (Universitäres Herz- und Gefäßzentrum Hamburg, Hamburg, Germany), Ahmed Farah (Zentralklinik Bad Berka, Bad Berka, Germany), Steven Worthley (Royal Adelaide Hospital, Adelaide, Australia), John Ormiston (Mercy Angiography, Auckland, New Zealand).
Data Safety Monitoring Board: Farrell Mendelsohn (Cardiology PC, Birmingham, AL, USA), William Gray (Columbia University Medical Center, New York, NY, USA), Daniel Clair (Cleveland Clinic Foundation, Cleveland, OH, USA).