Abstract
Aims: The aim of this study was to test the safety and performance of the Symplicity™ multi-electrode radiofrequency renal denervation system which was designed to reduce procedure time during renal denervation.
Methods and results: The multi-electrode radiofrequency renal denervation system feasibility study is a prospective, non-randomised, open label, feasibility study that enrolled 50 subjects with hypertension. The study utilises a new renal denervation catheter which contains an array of four electrodes mounted in a helical configuration at 90 degrees from each other to deliver radiofrequency energy simultaneously to all four renal artery quadrants for 60 seconds. The protocol specified one renal denervation treatment towards the distal end of each main renal artery with radiofrequency energy delivered for 60 seconds per treatment. Total treatment time for both renal arteries was two minutes. The 12-month change in office systolic blood pressure (SBP) and 24-hour SBP was -19.2±25.2 mmHg, p<0.001, and -7.6±20.0 mmHg, p=0.020, respectively. There were three patients with access-site complications, none of which was related to energy delivery; all were treated successfully. No new renal artery stenosis or hypertensive emergencies occurred.
Conclusions: The Symplicity multi-electrode radiofrequency renal denervation system was associated with a significant reduction in SBP at 12 months and minimal complications whilst it also reduced procedure time. Trial registration: NCT01699529
Introduction
Percutaneous transcatheter renal denervation (RDN) was first established with the single-electrode Symplicity™ renal denervation system (Medtronic, Inc., Santa Rosa, CA, USA). The SYMPLICITY HTN-1 and SYMPLICITY HTN-2 clinical studies used this single-electrode Symplicity™ renal denervation catheter to treat subjects with resistant hypertension and reported a reduction in office systolic blood pressure (BP) in the order of 25 mmHg at six months1,2, with results sustained at three years3-5. The SYMPLICITY HTN-3 multicentre trial was the first randomised, blinded, sham controlled study of RDN6. The study met its primary safety endpoint (with a major adverse event rate of 1.4% at six months, which was well below the performance goal of 9.8%, p>0.001)7. However, the six-month reduction in office systolic BP was –14±24 mmHg in the RDN group vs. –12±26 mmHg in the sham procedure group, and did not meet the primary efficacy endpoint (p=0.26 for the pre-specified analysis that required a 5 mmHg superiority margin)7. Factors which have been identified which may have confounded these outcomes include the patient population studied, issues with medication adherence, and the denervation procedure itself, such as lack of delivery of energy to all four quadrants of each renal artery7.
The next-generation Symplicity Spyral™ multi-electrode renal denervation catheter (Symplicity Spyral catheter) and associated Symplicity G3™ renal denervation radiofrequency generator (Symplicity G3 generator) (both Medtronic) were developed with the design goals of reducing treatment and procedure time, delivering a predetermined circumferential ablation pattern along the length of the renal artery, and improving ease of use while providing a safe and effective treatment for lowering BP.
In the present manuscript we present the six and twelve-month outcomes. This study was initiated to understand the safety of delivering unipolar RF energy simultaneously to four electrodes, and it specifically limited treatments to a maximum of two treatments (i.e., one four-electrode treatment per artery). CE mark was obtained in October 2013 for the new-generation Symplicity Spyral catheter and Symplicity G3 generator. It is important to note that, unlike this protocol, multiple four-electrode treatments per artery are allowed as part of the instructions for use of the commercial device.
Methods
TECHNICAL DESCRIPTION
The distal end of the Symplicity Spyral catheter consists of a spiral-shaped, self-expanding nitinol element onto which four electrodes are mounted. When deployed, the electrodes are at 90 degrees orthogonally from each other to cover all four quadrants of the artery’s circumference in a helical fashion. Sakakura et al reported that >75% of sympathetic nerves are located within a distance of 4.28 mm from the lumen as observed in cadavers8. Preclinical animal research established that the RF energy with a Symplicity multi-electrode renal denervation system ablates approximately 4 mm into the peri-arterial tissue of the main renal artery (data on file with Medtronic, Minneapolis, MN, USA).
The distal end of the catheter is designed to provide uniform electrode-arterial wall contact in vessels ranging in diameter between 3 mm and 8 mm. Thus, only one catheter is needed to complete a bilateral denervation procedure as it performs in a wide range of anatomies. The four electrodes simultaneously deliver radiofrequency (RF) energy for 60 seconds, thereby reducing treatment time. The design also allows for continuous blood flow throughout the treatment period to provide cooling to the endothelial and medial layers of the vessel to minimise thermal injury to the most luminal aspect of the renal artery wall.
The Symplicity Spyral catheter is compatible with a 0.014” coronary guidewire to help navigate the device in the anticipated tortuosity seen in renal arteries in humans with hypertension. The electrode array adopts a linear configuration when the guidewire is inserted into the distal end of the catheter. The electrodes deploy into a spiral (helical) shape upon retracting the guidewire into the catheter shaft due to the physical properties of nitinol, which exhibit superelastic and shape memory9. This self-expanding helical design conforms to the vessel’s natural shape and provides uniform apposition of the electrodes against the artery wall.
The catheter tip is designed to be non-traumatic and to direct the guidewire away from the vessel wall. A radiopaque marker is embedded in the catheter tip to provide guidance to the operator during catheter manipulations.
RF energy delivery with the Symplicity G3 generator utilises the safety algorithms and operating principles developed for the previous Symplicity G2™ generator (Medtronic). The generator monitors impedance and temperature values to detect electrode movement. In addition, the generator monitors temperature values and adjusts power delivery when needed to maintain these values within acceptable boundaries. Alternatively, the generator automatically turns off electrode(s) when certain boundary conditions are suddenly encountered; this is designed to protect the tissue against overheating.
STUDY POPULATION
The multi-electrode radiofrequency renal denervation system feasibility study is a prospective, single-arm, non-randomised, open label study that enrolled 50 subjects at four sites in Australia and New Zealand. Subjects included had uncontrolled hypertension as defined by office systolic BP ≥160 mmHg (≥150 mmHg for subjects with type 2 diabetes mellitus) despite adherence to a stable antihypertensive regimen of ≥3 drug classes (preferably including a diuretic) for a minimum of two weeks. There was no requirement that the antihypertensive medications be at maximum tolerable dose, and there was no inclusion or exclusion ambulatory blood pressure monitoring (ABPM) requirement. Thus subjects with pseudoresistant hypertension were included. Exclusion criteria included an estimated glomerular filtration rate (eGFR) of <45 mL/min/1.73 m2, type 1 diabetes mellitus, renal artery stenosis of >50%, renal artery aneurysm, and prior renal artery intervention. Main arteries were excluded if the treatable length (i.e., the length of artery free of visible anatomic abnormality or atheroma) was not at least 22 mm for a 4 to 5 mm diameter and at least 18 mm for a 7.1 to 8 mm diameter artery.
PROTOCOL
The protocol specified only one 60-second RDN treatment of four simultaneous ablations per artery to be applied towards the distal end of the vessel. Subjects were followed at one, three, six and twelve months post RDN, and will continue to be followed yearly to three years post RDN.
Key safety measures were: 1) acute procedure complications; 2) significant stenosis found on renal artery duplex ultrasound evaluation conducted at six months after RDN; 3) renal function assessed by calculation of eGFR using the Modification of Diet in Renal Disease (MDRD) calculation10 at baseline and through all follow-up visits. An in-hospital major adverse event was defined as mortality, new onset end-stage renal disease, significant embolic event resulting in end-organ damage, renal artery perforation or dissection requiring intervention, vascular complications requiring intervention, or hypertensive crisis/emergency. Key effectiveness measures included change in office and 24-hour ambulatory blood pressure, and change in medication use. ABPM was measured at six and twelve months (but not at one or three months).
Treatment time was defined as the cumulative time of RF energy delivery. Procedure time was defined as the time from RDN catheter insertion to guide catheter removal. Procedure success was defined as successful delivery of any RF energy in the absence of an in-hospital major adverse event. The ABPM monitor measured blood pressure every 15 minutes during the day and every 30 minutes at night, but, while the protocol did not specify ABPM acceptability requirements, the ABPM analysis excludes patients who did not have at least one daytime and at least one night-time reading reported at each follow-up visit. Pseudoresistance was defined as both office systolic BP ≥160 mmHg and 24-hour ambulatory systolic BP <135 mmHg.
An independent clinical events committee (CEC) adjudicated protocol-specified adverse events. Study monitors verified all patient source data at all follow-up time points.
STATISTICAL ANALYSIS
Continuous data were presented as mean±standard deviation (SD). Blood pressure and heart rate measurements at trial milestones were compared with pre-procedure measurements using the paired t-test. A change was considered significant if the two-sided alpha level was ≤0.05. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
CLINICAL CHARACTERISTICS
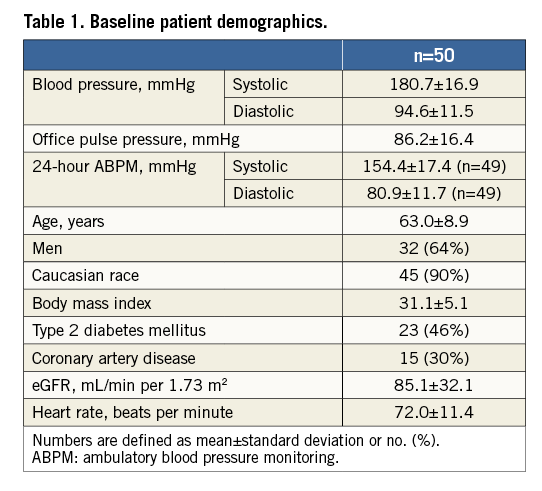
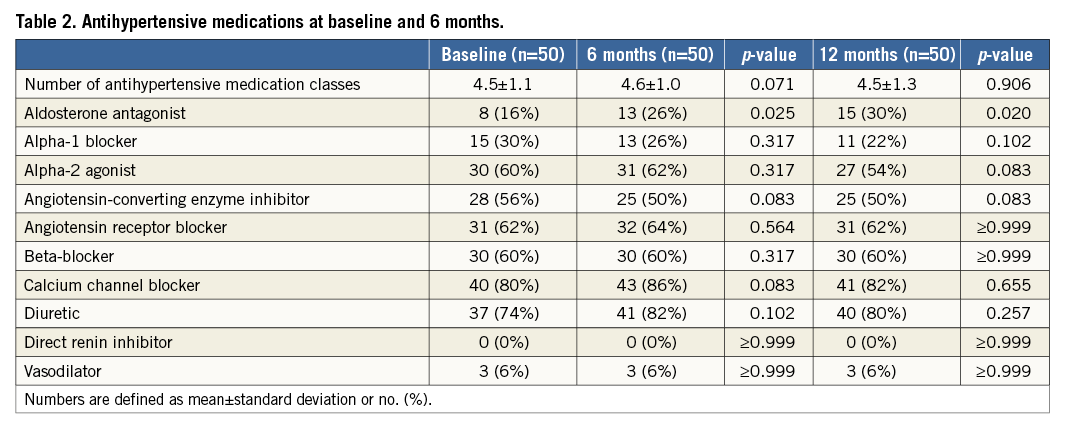
The baseline characteristics of the study subjects are presented in Table 1. The mean age was 63±9 years, 64% were men, 46% had type 2 diabetes mellitus, and baseline eGFR was 85±32 mL/min/1.73 m2. Baseline systolic and diastolic BP was 180.7±16.9 mmHg and 94.6±11.5 mmHg, in the office, and 154.4±17.4 mmHg and 80.9±11.7 mmHg during 24-hour ABPM, respectively. The mean number of antihypertensive drug classes was 4.5±1.1 (Table 2).
RENAL DENERVATION PROCEDURE
The device success rate was 100%. The total number of complete 60-second ablations was 6.5±1.5 per patient. Treatment time was two minutes. Procedure time was 22.6±11.2 minutes and mean contrast use was 116.8±50.5 cc.
Procedure success was 96% (48/50) due to two pseudoaneurysms at the vascular femoral access site (one patient also had an access-site haematoma), neither of which was related to energy delivery. One additional patient had a haematoma after discharge. Both haematomas were successfully treated with manual compression.
BLOOD PRESSURE
Office blood pressure was significantly reduced at three, six, and 12 months (Figure 1). At six months, systolic BP and diastolic BP were reduced by –19.9±25.0 mmHg, and –7.3±11.5 mmHg, respectively (p<0.001 for both), and were sustained at 12 months (–19.2±25.2 mmHg and –7.0±12.2 mmHg, respectively, p<0.001 for both). At 12 months, 65% (31/48) of the subjects achieved a reduction in systolic BP of ≥10 mmHg. At 12 months, the percent of subjects with controlled systolic BP (≤140 mmHg) was 17%, and the percent of subjects with SBP ≥180 mmHg was reduced to 23% from 46% at baseline, p=0.020 (Figure 2).
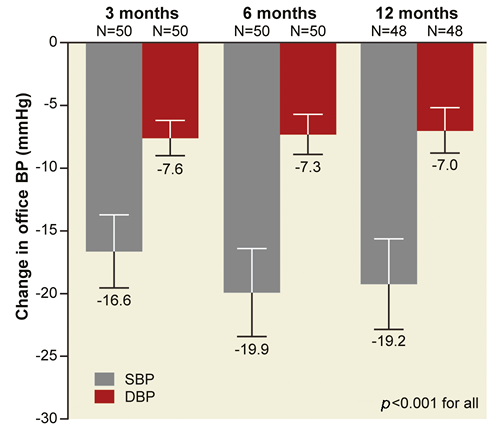
Figure 1. Change in office systolic and diastolic blood pressure at 3, 6 and 12 months. Numbers reported as mean±standard error. DBP: diastolic blood pressure; SBP: systolic blood pressure
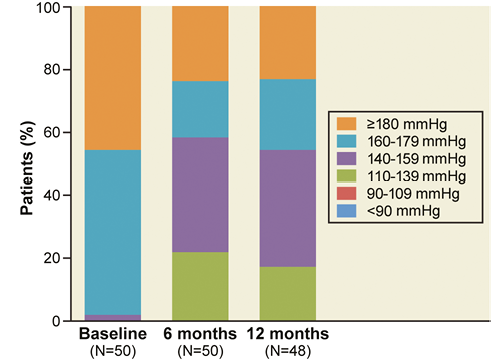
Figure 2. Distribution of office systolic blood pressure at baseline, 6, and 12 months.
The change in 24-hour systolic and diastolic BP by ABPM at six months was –5.6±17.2 mmHg (p=0.031) and –4.0±11.6 mmHg (p=0.022) (n=47), respectively, and sustained to 12 months, –7.6±20.0 mmHg (p=0.020) and –5.3±12.9 mmHg (p=0.012), respectively (n=41). Five of the subjects had pseudoresistance at baseline. When excluding these subjects, the reduction in 24-hour ambulatory systolic BP at 12 months was –9.8±20.2 mmHg, p=0.006 (n=36), and the change in office systolic BP was –20.5±25.8 mmHg, p<0.001 (n=42).
MEDICATIONS
The mean number of antihypertensive drug classes did not change from baseline to 12 months (p=0.906 vs. baseline) (Table 2). However, there was a significant increase in the number of patients who were prescribed aldosterone antagonists (16%, n=8, at baseline vs. 30%, n=15, at 12 months, p=0.020). This was offset by non-significant reductions in the use of alpha-1 blockers, alpha-2 agonists, and angiotensin-converting enzyme inhibitors.
SAFETY
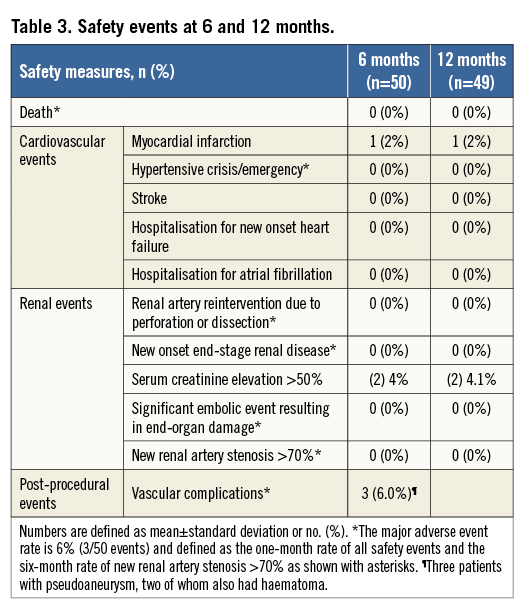
Table 3 provides safety outcomes. All subjects received renal artery duplex ultrasound evaluation at six months, and no new significant renal artery stenoses were found. At 12 months, there were no hypertensive crises, no deaths, and no new onset of end-stage renal disease. Therefore, the major adverse event rate at 12 months was 6% (3/50) and comprised the three patients with periprocedural access-site vascular complications. While two patients (4.1%) experienced a more than 50% rise in serum creatinine from baseline, there was no clinically meaningful change in renal function at 12 months compared to baseline when assessed using eGFR (81.2±32.0 mL/min/1.73 m2 at 12 months, a change of –4.1±14.9 mL/min/1.73 m2, p=0.066 vs. baseline).
Discussion
The Symplicity multi-electrode radiofrequency renal denervation system feasibility study was undertaken to evaluate the safety of delivering RF energy simultaneously from a multi-electrode unipolar catheter system when performing renal denervation. No safety events related to RDN itself were reported and the procedure was shown to be safe. Three patients experienced post-procedural vascular complications, none of which was related to RF energy delivery. Similarly, a low adverse event rate has been observed across all the SYMPLICITY clinical studies1-3,7.
Additionally, a significant reduction in office systolic and diastolic BP at six months was observed (–19.9 and –7.3 mmHg, respectively, p<0.001 for both measurements) and sustained to 12 months (–19.2 and –7.0 mmHg, respectively, p<0.001 for both measurements). The change in 24-hour ambulatory BP was also significant at 12 months (–7.6±20.0 mmHg, p=0.020). When patients with pseudoresistance at baseline are excluded, the 12-month change in 24-hour ambulatory BP was –9.8±20.2 mmHg, p=0.006 (n=36). This change in BP occurred despite limiting treatment to one denervation per renal artery with no change in the overall number of antihypertensive medication classes. (There was a significant increase in aldosterone antagonist use but this was offset by reductions in other antihypertensive classes).
In this study, the protocol limited renal denervation to one treatment per artery in order to test catheter and generator performance. In addition, the protocol specified that the ablation should be performed in the distal end of the main renal artery. This was pre-specified due to recent studies using human cadaver kidneys which reported greater axon proximity to the vessel lumen in the distal renal main artery8 as well as preclinical studies reporting greater denervation success as assessed by cortical norepinephrine depletion and axon destruction with distal main renal artery denervation.
In a post hoc matched cohort analysis in the SYMPLICITY HTN-3 study, more ablations were associated with a greater reduction in office systolic blood pressure at six months after RDN (p-value for trend=0.01). In this study, the operator could not repeat treatment where an electrode automatically turned off, which occurs when boundary conditions of temperature or impedance are detected as part of the safety algorithms in the generator. Patients in this study thus received an average of 6.5 ablations in both renal arteries out of the possible eight complete 60-second ablations. This indicates that, on average, one electrode automatically turned off during treatment when treating either the left or right side, and thus patients did not uniformly receive four-quadrant coverage bilaterally. Multiple ablations in a helical fashion might help to ensure that four-quadrant ablations are obtained. The CE-marked Symplicity Spyral catheter instructions for use allow for multiple treatments per artery and recommend performing the first series of ablations proximal to the first major renal artery bifurcation. The operator can also choose to deselect any of the four electrodes for any treatment.
Given that renal artery nerves are located circumferentially around the artery8,11, the Symplicity multi-electrode renal denervation catheter was designed to deliver RF energy in a circumferential pattern that extends along the length of the renal artery. Preclinical animal research documented that ablation from each of the four electrodes spans approximately 20-25% of the circumference of the vessel12. Thus, by positioning the four electrodes in four distinct quadrants, energy reaches nerves located in each quadrant around the artery. Time is saved not only by providing simultaneous ablation with four electrodes, but also by allowing RDN to be performed using a single angiographic projection. Additionally, the 4 Fr catheter can be used with a 6 Fr guide catheter to treat a wide range of vessel diameters (between 3 mm and 8 mm) without the need for vessel sizing. These design characteristics resulted in a procedure time of 23 minutes which was probably increased artificially by study-specified activities.
The study is limited by the relatively small sample size, and the single-arm, non-randomised, open label design; however, this is common for feasibility studies. The protocol did not exclude subjects with pseudoresistant hypertension, and the use of aldosterone antagonists was low at enrolment. Additionally, the protocol limited delivery of energy to each renal artery once, with a resultant low total number of ablation sites per patient. Allowance to deliver energy multiple times in each artery where the artery length allowed it would have resulted in a higher total number of ablation sites per patient, possibly impacting on efficacy. Despite this, the 6.5 distal ablations per subject resulted in a substantial reduction in systolic BP.
Conclusion
The multi-electrode radiofrequency renal denervation system feasibility study confirms the safety of treating patients with uncontrolled hypertension with the Symplicity multi-electrode radiofrequency renal denervation system. Minimal safety complications were reported, none of which was directly related to the delivery of RF energy. Treatment time was reduced to two minutes. The catheter provided improved ease of use and shortened total procedure time, and reduced overall the contrast required. Despite limiting treatment to one four-electrode treatment per artery and allowing for patients with pseudoresistant hypertension, the study reported a significant reduction in office systolic blood pressure at 12 months.
The multi-electrode radiofrequency renal denervation system feasibility study is the first study to examine the safety and performance of the Symplicity multi-electrode radiofrequency renal denervation system. The Symplicity Spyral catheter is commercially available and is also being studied in the Global SYMPLICITY registry, which will provide real-world outcomes, and allow evaluation of outcomes after multiple treatments per artery, after excluding patients with pseudoresistant hypertension, and focusing on treatments directed both in the distal segment of the main artery and possibly in the renal artery branches.
| Impact on daily practice Physicians treating patients with treatment-resistant hypertension with renal denervation prefer a shorter procedure time and greater assurance to obtain four-quadrant energy delivery more easily compared with that required with a single-electrode catheter. The Symplicity Spyral™ multi-electrode renal denervation catheter delivers RF energy simultaneously from four electrodes to shorten RF treatment time to 60 seconds and can treat vessel diameters between 3 and 8 mm. The four electrodes are located in a spiral configuration at 90 degrees from each other, thereby providing treatment at all four quadrants of the artery wall. This study of 50 patients treated with the Symplicity™ multi-electrode radiofrequency renal denervation system supports the safety of this novel device. It also reported a significant reduction in office systolic blood pressure at 12 months. |
Acknowledgements
K. Pratt and L. Ruehlow provided clinical support. N. Brilakis, C. Gilbert and S. Cohen provided editorial support. M. Fahy and M. Yun provided statistical support (all employees of Medtronic).
Funding
Medtronic, Minneapolis, MN, USA.
Conflict of interest statement
R. Whitbourn has received small honoraria from Medtronic, and the institution has received research grants from Medtronic. S. Harding has received honoraria from Medtronic, and is an advisor to Medtronic. A. Walton has received honoraria from Medtronic, and is an advisor to Medtronic.
References

