
Abstract
Aims: To evaluate in a preclinical model the utility of a monopolar electrode catheter delivering radiofrequency (RF) energy placed into the renal pelvis in order to treat resistant hypertension (RH).
Methods and results: Sixteen female domestic swine weighing 60-65 kg underwent renal pelvic denervation via ureteral access. Three animals were euthanised immediately after delivery of RF energy; five animals were allowed to survive for seven days, six animals were allowed to survive for 14 days and two animals were allowed to survive for 30 days. Renal cortical norepinephrine levels were measured in all groups of animals. Histopathology of the treated zone was performed to confirm nerve damage. Renal cortical tissue was harvested for determination of tissue norepinephrine by HPLC. The kidneys were then profusion-fixed and harvested for histopathologic analysis. Mean reduction of norepinephrine levels was 60.4% compared to control. Histopathology confirmed nerve ablation in the treated zone.
Conclusions: In this small, preclinical study, we introduce a new non-vascular system to treat resistant hypertension. If the current clinical experience confirms efficacy and safety, this approach may be one way to treat patients who cannot be treated with the standard percutaneous arterial devices.
Introduction
Hypertension affects more than a quarter of adults in developed societies1-4. The global burden of hypertension has been predicted to grow from 972 million in 2000 (26.4% of the population) to 1,560 million by 2025 (29.2% of the population)1. Cardiovascular mortality risk doubles with each 20 mm increase in systolic blood pressure5,6.
Patients thought to be candidates for renal denervation (RDN) include those with resistant hypertension (RH) as defined by the American Heart Association7. Resistant hypertensive patients are defined as individuals who have failed to achieve target blood pressure (less than 140/90 mmHg, or less than 130/80 mmHg in patients with diabetes mellitus or chronic kidney disease) despite concomitant full dose use of three antihypertensives, one of which should be a diuretic. Other definitions include controlled blood pressure that enhances renin release, systemic sympathetic neural stimulation and renal artery vasoconstriction8. Surgical interruption of the sympathetic nervous system has been shown to effectively treat hypertension9. However, this procedure has not been well accepted due to the many adverse effects which include bowel and bladder incontinence, impotence, syncope, orthostatic hypotension and prolonged hospitalisation.
RDN has been effective in 85% of patients with follow-up to two years8. One downside of intra-arterial systems is that they require a fairly normal renal anatomy as well as the absence of renal aneurysms or renal stents. Renal arteries are not always easily cannulated given that the renal artery diameter may vary between 2 mm and 8 mm. Not all vessels can be treated with the currently available devices. One of the devices, the Medtronic Symplicity device (Medtronic, Minneapolis, MN, USA), has a procedural time greater than 45 minutes, which can result in increased exposure to contrast and radiation.
RDN procedural outcomes may not be known until at least 15 days of follow-up, and more recent studies suggest as many as 20%-30% of patients are non-responders9-13. It is not clear if this non-response is due to a lack of destruction of sufficient efferent or afferent nerve fibres or other factors. Intra-arterial renal denervation is believed to affect primarily efferent nerves.
The Verve Medical device (Verve Medical, Santa Barbara, CA, USA) is a non-vascular system that exploits the proximity of the renal nerves to the renal pelvis. It has been shown that in this location both efferent and afferent nerves are located and intertwined within the multiple layers of the renal pelvic wall14.
The system is a proprietary radiofrequency catheter system which can be introduced transurethrally to the target treatment area, the renal pelvis, with standard urologic techniques. This procedure is not restricted by anatomic variances of the artery, and may not necessitate utilisation of contrast media. If contrast is administered, it will be delivered in a retrograde manner and not systemically.
The system consists of a monopolar radiofrequency electrode catheter and a radiofrequency generator which monitors and regulates power, temperature, time and impedance. The 9 Fr catheter consists of a hollow lumen to track over a standard 0.035” guidewire for placement into the target area. The electrode array is located at the distal end of the catheter and radially expands to contact and dilate the renal pelvis. Low power, monopolar energy is delivered from the generator through the electrodes into the renal pelvic wall ablating the residing afferent and efferent nerves (Table 1, Figures 1-3).
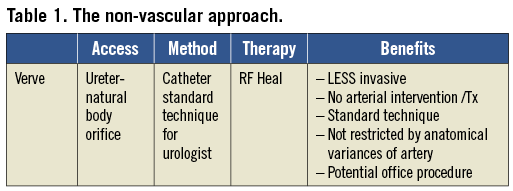

Figure 1. The radiofrequency generator used for the Verve Medical device.

Figure 2. The monopolar radiofrequency electrode catheter for delivery to the renal pelvis.
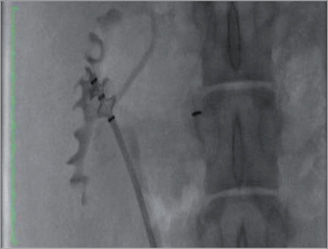
Figure 3. Fluoroscopic image of the device in a swine’s renal pelvis.
Methods
Sixteen female domestic swine weighing 60-65 kg underwent renal pelvic denervation via ureteral access. Three animals were euthanised immediately after delivery of RF energy; five animals were euthanised at seven days, six animals at 14 days and two animals were euthanised at 30 days. Renal cortical norepinephrine levels were measured in all groups of animals. Histopathology of the treated zone was performed to confirm nerve damage.
Animals were fasted overnight, then sedated with ketamine, intubated and maintained on isoflurane anaesthesia throughout the procedure. A ventral midline laparotomy was performed to expose the urinary bladder. A 5 cm incision was made in the ventral aspect of the bladder to access the ureteral orifice. The Verve Medical device was passed retrograde over a 0.035” guidewire from the bladder to the renal pelvis and RF ablation was performed. The procedure was repeated in the contralateral kidney. In two animals, RF energy was not applied to the contralateral kidney (sham procedure). The urinary bladder and laparotomy were closed and the animals were allowed to recover from anaesthesia. Bilateral pyelogram, ureterogram and renal angiography were performed prior to euthanasia in two of the seven-day animals, and all of the animals allowed to survive out to 14 and 30 days. Immediately following euthanasia, renal cortical tissue was harvested for determination of tissue norepinephrine concentration by high performance liquid chromatography (HPLC). The kidneys were then perfusion-fixed and harvested for histopathologic analysis (Figure 4).
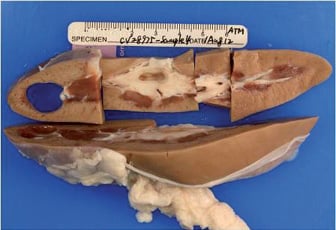
Figure 4. Gross pathology of the explanted kidney of a swine.
Results
Animals returned to a normal diet and uresis the next day. Follow-up pyelography, ureterography and renal arterial angiography were unremarkable. In all animals, there was a reduction of norepinephrine levels compared to control samples. Mean reduction of norepinephrine levels was 60.4% compared to control. Histopathology confirmed nerve ablation in the treated zone with no parenchymal or vascular thermal injury (Figure 5).
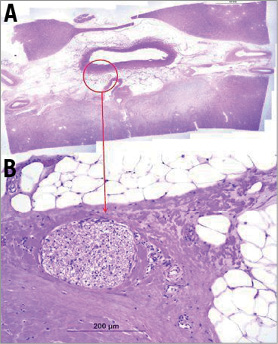
Figure 5. (A) Image shows the relation to the surrounding renal pelvis tissue. Not all nuclei are damaged. (B) High power showing severe nerve injury with necrosis of endoneural cells and perineural thermal injury. Not all nuclei are damaged.
Discussion
Ensuing human trials will need to confirm the efficacy of the Verve Medical system. The device may allow treatment of patients with arterial anatomic abnormalities. For instance, only 55% of patients have a single hilar renal artery15. Limitations of the current vascular approaches for RDN include the possibility of renal artery anomalies, inappropriate vessel size or vessel origination from the aorta, previous stents, aneurysms, bifurcated renal arteries or renal insufficiency.
The treatment of patients who have renal insufficiency that precludes contrast utilisation during the procedure as well as the treatment of patients with stent placements are unique advantages of an alternative treatment system as proposed in this paper. It is of interest to consider applications in patients who otherwise are not candidates for renal denervation at this time. In the general hypertensive population, patients who do not achieve blood pressure control are between 28% to 53.1% of cases1, and future applications could be in patients with mild hypertension only, heart failure medication intolerant patients, diabetic patients, patients with sleep apnoea or even patients with metabolic syndrome. In contrast to the Medtronic Symplicity device, the Verve Medical device does not require the operator to reposition the ablation catheter to multiple sites for treatment. It is possible that only a single ablative treatment in each renal pelvis is needed. The device is delivered using standard urologic techniques and the procedure could be performed with cystoscopy and ultrasound in an outpatient or possibly office setting.
It is conceivable that the treatment of efferent and afferent nerves in close proximity to this particular catheter may require less energy delivery, with lower temperatures than current RF, ultrasound or other energy-based systems. It is also possible that the monopolar RF catheter may also reduce procedure time as compared to arterial delivery systems. Because the device treats the nerves within the pelvis, which are predominantly afferent nerves16, there may be a more direct effect on the sympathetic nervous system in patients with pelvic directed RDN (Figure 6).
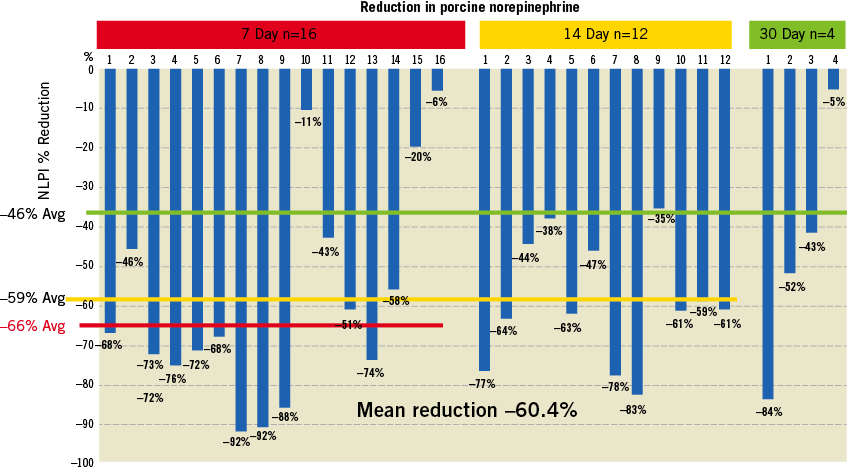
Figure 6. Reduction from baseline of norepinephrine in the swine model post-intrapelvic RF ablation. Note that the data are per kidney.
Some clinical limitations of this new technique need to be described. Patients with nephrolithiasis, pelvic ureteral junction (PUJ) obstruction, previous renal surgery and other obstructive/reflex uropathies, ascending urinary tract infections as well as patients with indwelling catheters probably would not be considered candidates for this approach.
In general terms, renal sympathetic efferent nerve activity is involved in renal excretion, sodium retention, reduced renal blood flow and peripheral and cardiovascular effects. Renal sympathetic afferent nerves affect the central nervous drive; therefore, the inhibition of renal afferent nerves is a potential target for RH and carries with it the promise that control may result in a subsequent decline in associated cardiovascular morbidity/mortality. Targeted afferent ablation could not only reduce blood pressure, but could ameliorate organ-specific damage caused by chronic sympathetic overactivity.
Conclusion
Although numerous articles show renal interruption of the renal afferent nerves will attenuate systemic tone lowering blood pressure, most studies have primarily included treatment of efferent systems. In contrast to the widespread distribution of efferent sympathetic nerve fibres in the kidneys15, the majority of the afferent renal sensory nerves are located in the renal pelvic area16.
As many as 44% of patients in the Symplicity HTN-1 and 2 trials were excluded because of clinical and anatomic study entry requirements17. If clinical experience with the Verve Medical device confirms efficacy and safety, this approach may be one way to treat patients who cannot be treated with the standard percutaneous arterial devices.
Acknowledgements
The authors would like to thank and acknowledge Peggy Layman for the manuscript project coordination and research, revisions and submission, as well as CV Path, for histopathology work and Constantine Davlantes.
Conflict of interest statement
R. Heuser is a stockholder of Verve Medical. T. Buelna is President and CEO of Verve Medical and W. Berci is Chief Business Officer of Verve Medical. B. Hubbard is a consultant to Verve Medical. All other authors have no conflicts of interest to declare.

