
Abstract
Aims: The blood pressure-lowering effect of percutaneous renal denervation (RDN) is controversial. The success of RDN may be device-dependent. We sought to compare the efficacy of RDN by chemical neurolysis using alcohol (Peregrine System Infusion Catheter; Ablative Solutions, Inc., Menlo Park, CA, USA) to RDN by radiofrequency (RF) ablation with the single-electrode RF catheter (Symplicity Flex; Medtronic, Minneapolis, MN, USA) in a porcine model.
Methods and results: This was a prospective, randomised, blinded study. Pigs were assigned to undergo bilateral RF ablation or chemical neurolysis. Primary endpoints were ablation depth and renal tissue norepinephrine (NE) concentrations at three-month follow-up. Twelve pigs underwent RF ablation (n=4) or chemical neurolysis by infusion of 0.3 mL (n=4) or 0.6 mL (n=4) alcohol. Ninety days after RF ablation and chemical neurolysis with 0.3 mL and 0.6 mL of alcohol, mean maximal tissue injury depth was 3.9±1.2 mm, 6.6±1.7 mm and 8.2±2.2 mm, respectively (p<0.001 for either dose of alcohol vs. RF ablation). Compared with historical controls, median renal tissue NE concentration reductions were 66%, 78% and 83% after RF ablation and chemical neurolysis using 0.3 mL and 0.6 mL alcohol, respectively (p=0.107 for chemical neurolysis vs. RF ablation). Mean total ablation area was significantly greater in both (0.3 mL and 0.6 mL) alcohol groups (p=0.0001 for both) than the RF ablation group (30.8±13.7 mm2, 41.6±12.4 mm2 and 11.0±7.5 mm2, respectively).
Conclusions: RDN is more effective using chemical neurolysis than single-electrode RF ablation. Our findings suggest that the efficacy of RDN may be device-dependent.
Introduction
We sought to compare the efficacy of RDN using chemical neurolysis by percutaneous perivascular alcohol infusion (Peregrine System™; Ablative Solutions Inc., Palo Alto, CA, USA) to RDN with radiofrequency ablation using the Symplicity Flex™ System (Medtronic, Minneapolis, MN, USA) in a porcine model.
Methods
This was a prospective, randomised, blinded study. Twelve Yorkshire pigs were randomised in a 1:1:1 fashion to undergo bilateral RDN using the Symplicity Flex catheter (n=4) or chemical neurolysis using the Peregrine System with infusion of either 0.3 mL (n=4) or 0.6 mL of alcohol (n=4). Randomisation to treatment groups was performed after the baseline angiogram was obtained and the length of the renal arteries measured. If both renal arteries were ≥20 mm, the animal was randomised to any of the three groups. If an artery was not eligible for treatment with the Symplicity catheter, i.e., <20 mm, the animal was randomised to one of the alcohol groups. While the randomisation scheme was modified based on the above stated exclusion for the RF device (renal artery length <20 mm), the authors believe that this modification was not likely to have affected the outcomes reported.
The study was conducted under the general principles of the Good Laboratory Practice regulations. The protocol for this study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the test facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and licensed by the United States Department of Agriculture (USDA).
Animals were pre-medicated with 325 mg of aspirin, 30 mg of nifedipine and 75 mg of clopidogrel by mouth once daily for two days before the procedure.
PROCEDURE
Selective renal angiography was performed before and immediately after the procedure.
RDN using the Symplicity Flex catheter was performed as previously described1, with four ablations per renal artery approximately equally distributed between the renal artery ostium and bifurcation by a single operator experienced in renal denervation using the radiofrequency catheter.
RDN using the Peregrine System (Figure 1) was performed as previously described2 by one operator without experience of using this device (n=7) and one operator with prior experience of using this device (n=1).
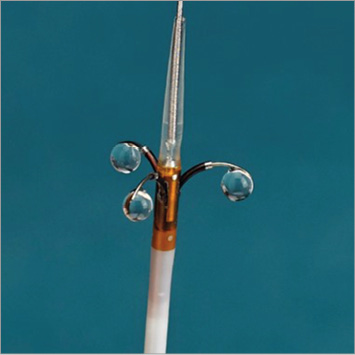
Figure 1. Peregrine System.
FOLLOW-UP
At 45 and 90 days post procedure, renal angiography was performed. Immediately after final angiography, a midline laparotomy was performed. After evaluation of the retroperitoneal space and adjacent tissues, the kidneys were exposed and isolated. Using a 10 mm biopsy punch, four samples were collected from each of three sections of each kidney: proximal pole, mid body and distal pole (a total of 12 samples per kidney). Samples were placed in cryovials, weighed and flash frozen before being stored at –80°C. Six samples per kidney (two samples from each section) were evaluated for renal tissue norepinephrine concentration (RTNEC) using high-power liquid chromatography (HPLC) with electrochemical detection by an independent laboratory blinded to the procedure (BASi Preclinical Services, West Lafayette, IN, USA), and the remaining samples were kept as a back-up. Concentrations from each of the six sites from each kidney were then used to calculate the average per pig and averages and medians per study group. Control animal data from a historical group (n=13) of pigs (that did not undergo renal denervation) were used to provide control RTNECs. RTNECs for the control animals were generated at the same test facilities using the same methodologies as the treated animals. These analyses were also conducted in a blinded fashion.
A general necropsy was conducted. The renal arteries and kidneys were harvested en bloc. After the tissues were appropriately fixed, they were sent to an independent pathology lab (CVPath Institute Inc., Gaithersburg, MD, USA) for microscopic evaluation. The renal arteries were trimmed at three levels (Figure 2), and trimmed tissues were embedded in paraffin using standard techniques and stained with H&E and Movat’s Pentachrome. Multiple (6-11 sections, at 3-5 mm intervals) sections (5 microns in thickness) from each renal artery were evaluated microscopically. In addition, immunohistochemistry was performed on the sections by staining for tyrosine hydroxylase (TH) and neurofilament protein (NFP). All pathologists performing the evaluations were blinded to the treatment groups and were un-blinded only after gross and microscopic evaluations had been completed.
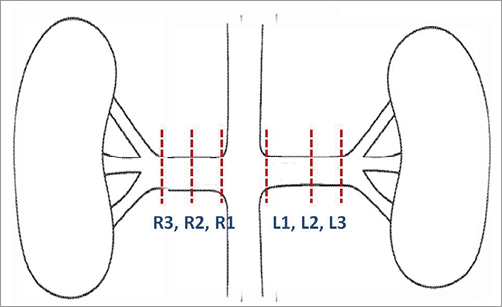
Figure 2. Levels of trimming.
Histologic evaluation included semi-quantification of endothelial loss, arterial and venous medial injury (depth and circumference), inflammation, degenerative changes and necrosis, renal artery medial injury and renal artery thinning, as described by Sakakura et al3. In addition, a semi-quantitative analysis of nerves around the renal artery using a quadrant analysis scheme, immunostaining (TH and NFP) and measurements of ablation distance from the lumen of the artery and ablation cross-sectional area was performed as described by Sakakura et al3.
ENDPOINTS
The primary endpoints were maximal ablation depth and tissue injury area as previously defined and RTNECs (averages of all six samples from each kidney per animal were used for comparison). In addition, the distribution of nerve damage and immunohistochemical effect on nerve structure and function were assessed.
STATISTICAL ANALYSIS
All values were expressed as mean±standard deviation. To assess maximal ablation depths and tissue injury area, ANOVA was used to test for differences among the three groups, using JMP software, version 5.0 (SAS Institute Inc., Cary, NC, USA). Post hoc comparisons were performed when the results of the one-way ANOVA test were significant (p<0.05), and statistical significance was adjusted by the Tukey-Kramer test. A value of p<0.05 was considered statistically significant.
As the normality assumption for RTNECs could not be confirmed, in order to assess RTNECs, the median (IQR) concentrations for each group were computed after averaging values across kidneys by subject. Groups were compared using the non-parametric Wilcoxon rank-sum test.
Results
BASELINE CHARACTERISTICS
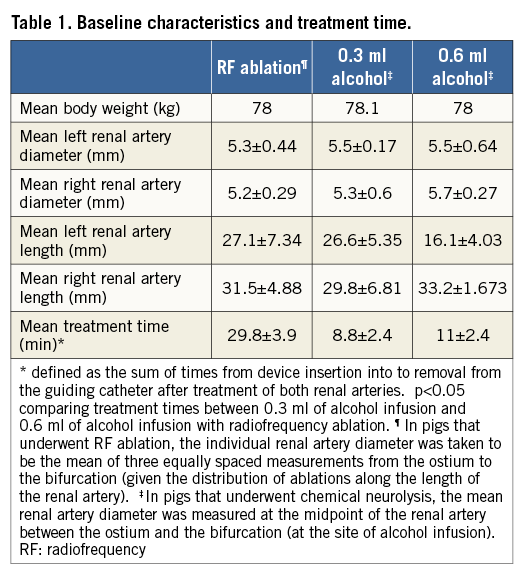
The average weight of the pigs and renal artery dimensions are outlined in Table 1.
PROCEDURE
Renal denervation was performed without difficulty using either device. Of note, treatment times were significantly shorter in animals treated with chemical neurolysis compared with RF ablation (p-values <0.05 for both, chemical neurolysis with 0.3 mL and 0.6 mL alcohol compared with RF ablation). The mean impedance drops for RDN with the RF catheter were 18.4±2.3%, 18.0±3.7%, 15.4±3.5% and 15.6±2.7% for the first, second, third and fourth denervations, respectively. There were no complications.
MORBIDITY AND MORTALITY
All 12 animals were recovered from the procedure without incident. No serious study-related morbidity or mortality was recorded for any animal. Eleven of the 12 animals survived to the intended endpoint and were clinically normal throughout the three-month survival period. One pig (included in this analysis) that had undergone chemical neurolysis with 0.3 mL of alcohol was euthanised at day 68 due to a non-study-related (foot) injury.
GROSS PATHOLOGICAL TERMINAL EVALUATION
The abdominal surface of the retroperitoneal lining and adjacent abdominal organs of all animals from all groups were normal. Each kidney was exposed and no abnormalities were noted within the retroperitoneal space. There were no accessory renal arteries in any of the pigs.
VASCULAR AND RENAL SAFETY
Immediately after the procedure, in pigs treated with RF ablation, mild to moderate notching (multifocal luminal irregularities), typically seen after RF ablation, was noted in seven of eight renal arteries. Mild to moderate spasm was also noted in four out of eight renal arteries treated with RF. In pigs treated with chemical neurolysis, minimal or mild focal luminal irregularities were seen in seven of 16 arteries and spasm in seven of 16 renal arteries (mild to moderate in six, severe in one). In one vessel treated with chemical neurolysis, a transient (<2 minutes) micro-leak of contrast was noted at routine immediate post-procedural angiography. At both the 45- and 90-day angiographic follow-up, all renal arteries from all groups appeared normal.
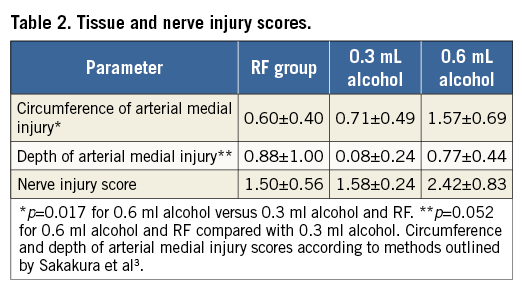
Histological examination demonstrated absence of endothelial cell loss or mural thrombus in all animals regardless of treatment group. Focal mild intimal thickening was observed in three renal arteries, two from the chemical neurolysis group using 0.6 mL alcohol and one from the RF ablation group. Pathological changes in the media of the renal arteries from all three groups were generally characterised by focal smooth muscle cell loss with proteoglycan replacement. The associated medial injury scores varied from 0 to 4 in depth and 0 to 2 in circumference. There was a trend towards a greater depth of arterial medial injury in the 0.6 mL and RF groups compared to the 0.3 mL alcohol group (p=0.052), and the circumference of arterial medial injury was significantly greater in the 0.6 mL alcohol group compared with the 0.3 mL alcohol and RF groups (p=0.017) (Table 2). There was no evidence of medial thinning or aneurysm noted in any section. Furthermore, medial damage, if present, was typically observed on the abluminal side in the animals from both of the alcohol groups. There was no evidence of arterial negative remodelling, reducing the cross-sectional area of the lumen. Four of the eight arteries from the RF group showed transmural medial damage, whereas in arteries from the alcohol groups, when injury was noted, it was abluminal and not transmural. Peri-arterial soft tissue showed mild to severe fibrosis, which represents the healing stage of the sclerotic reaction from the alcohol as well as the RF ablation. In sections where severe peri-adventitial fibrosis was noted, there was no evidence of vascular negative remodelling or constriction, or other unfavourable healing. Endothelial cell loss was not observed in the renal veins and there was no luminal thrombus. There were no significant differences among the three groups.
It should be noted that histopathologic evaluation of the kidneys of all animals was within normal limits.
PRIMARY ENDPOINTS
Mean maximal depth (distance) of tissue injury (ablation depth) from the vessel lumen was 3.9±1.2 mm for RF, 6.6±1.7 mm for 0.3 mL alcohol and 8.2±2.2 mm for 0.6 mL alcohol (p=0.0003 for the lower and higher dose of alcohol compared with RF ablation) (Figure 3). Mean total ablation area (morphometric analysis) was significantly greater in both the 0.3 mL and 0.6 mL alcohol groups (both groups p=0.0001) than with radiofrequency ablation, at 30.8±13.7 mm2, 41.6±12.4 mm2 and 11.0±7.5 mm2, respectively (Figure 4). Using morphometric analysis, in 12 of the 16 arteries treated with chemical neurolysis, tissue injury was circumferential, whereas circumferential tissue injury was not seen in any of the arteries treated with RF (Figure 5).
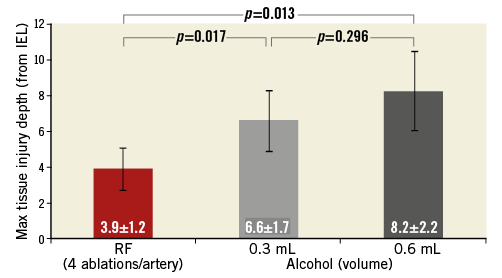
Figure 3. Mean maximal tissue injury depths (distance from lumen). RF: radiofrequency ablation
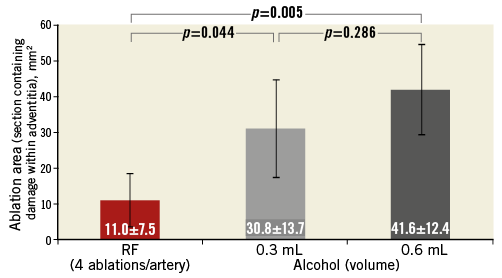
Figure 4. Mean ablation area (mm2). RF: radiofrequency ablation
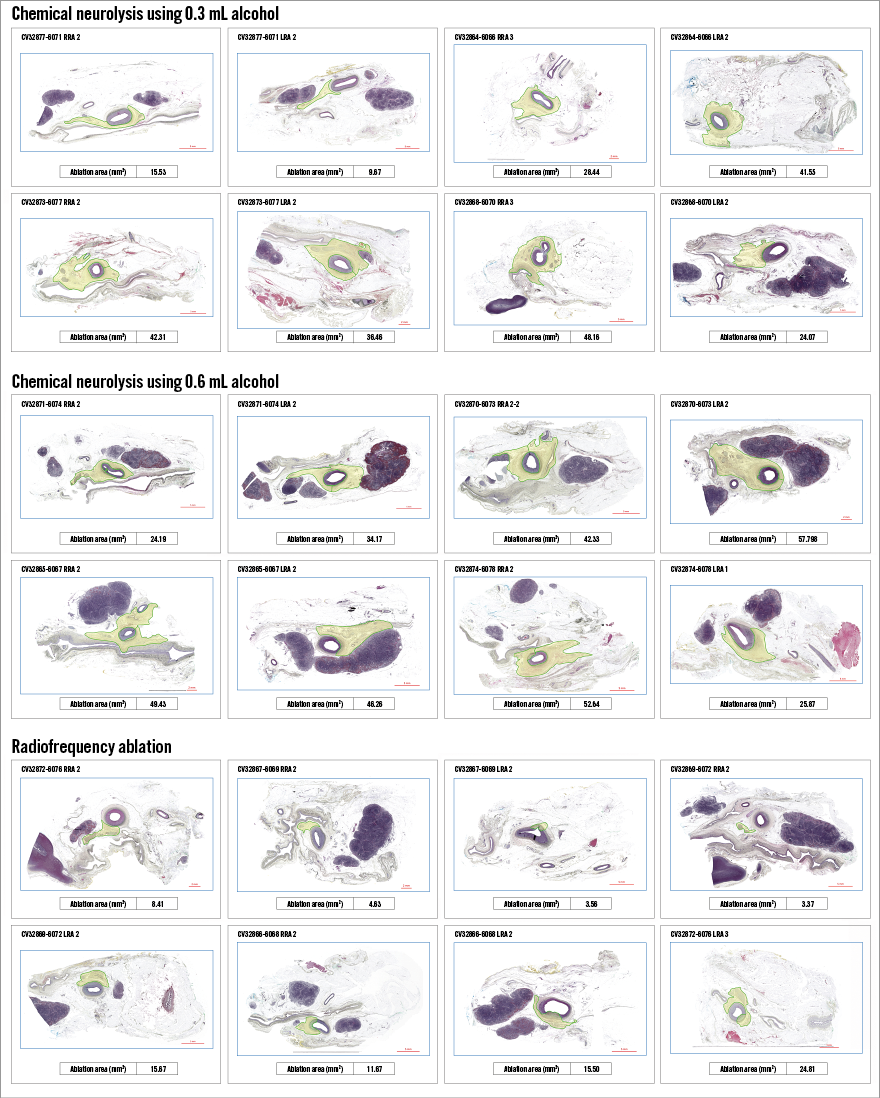
Figure 5. Morphometric analysis.
The median RTNEC was 286 ng/gr (mean: 298.0±75.0 ng/gr) in the control animals. For the animals treated in this study, median RTNECs were 97.0 ng/gr (mean: 101.2±36.5 ng/gr) after RF ablation, 68.5 ng/gr (mean: 65.0±32.0 ng/gr) after chemical neurolysis with 0.3 mL of alcohol and 47.5 ng/gr (mean: 64.4±39.0 ng/gr) after chemical neurolysis with 0.6 mL of alcohol (Figure 6). Hence, compared with control animals, there was a 66%, 78% and 83% reduction in median RTNECs with RF ablation and chemical neurolysis using 0.3 mL and 0.6 mL alcohol, respectively (p=0.039 for either 0.3 ml alcohol, 0.6 ml alcohol or RF vs. control). There was a trend towards lower RTNECs after chemical neurolysis compared with RF ablation (p=0.107). When the higher (0.6 mL) and lower (0.3 mL) amounts of alcohol infusion were compared separately with RF ablation, there was a trend towards lower RTNECs with either amount of alcohol infusion (p=0.19 for 0.3 mL alcohol vs. RF ablation, p=0.19 for 0.6 mL alcohol vs. RF ablation).
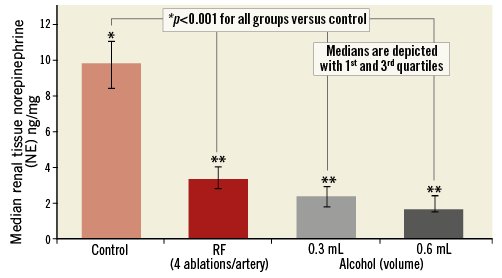
Figure 6. Renal nerve tissue norepinephrine; group medians. p=0.107 when radiofrequency ablation is compared to chemical neurolysis. **p=0.19 when radiofrequency ablation is separately compared to chemical neurolysis with 0.3 ml and 0.6 ml, respectively. NE: norepinephrine; RF: radiofrequency ablation
HISTOLOGY
Histopathologic sections were divided into four quadrants. Tissue injury was analysed and scored for each quadrant. Nerves were present equally in all three treatment groups. The averages per quadrant were: 3.3 for RF, 3.4 for 0.3 mL and 3.3 for the 0.6 mL group. There was an average of 0.10±0.07, 0.17±0.09 and 0.37±0.24 quadrants with nerve injury of 2 or greater in the RF, 0.3 mL, and 0.6 mL group, respectively. There was an average of 0.07±0.06, 0.05±0.09, and 0.22±0.18 quadrants with nerve injury of 3 or greater in the RF, 0.3 mL, and 0.6 mL group, respectively. The difference was overall statistically significant at post hoc analysis (Tukey-Kramer, p<0.05) for the 0.6 mL groups compared to RF and 0.3 mL at quadrants with nerve injury of 2 or greater and for the 0.6 mL group compared to 0.3 mL at quadrants with nerve injury of 3 or greater.
Overall, nerves showed mild to severe damage characterised by pyknotic nuclei, vacuolisation, and perineurium fibrosis. For the two doses of alcohol tested, typically the most pronounced damage to the nerves was in section 2 (mid vessel), i.e., at the single site where the alcohol was infused. Nerve damage was noted to extend to the proximal and distal sections of each renal artery as well, indicating spread of the treatment along the length of the vessel. For the RF group, the locations of nerve damage were more focal as compared to alcohol ablation, and may have depended on whether or not sectioning occurred at the ablation site. The mean nerve injury scores were 1.5, 1.6, and 2.4 for the RF, 0.3 mL, and 0.6 mL group, respectively. The overall difference was statistically significant (Tukey-Kramer, p<0.05), and pairwise comparisons showed that the nerve injury score was significantly greater in the 0.6 mL alcohol group than the 0.3 mL alcohol and RF groups (p=0.0099).
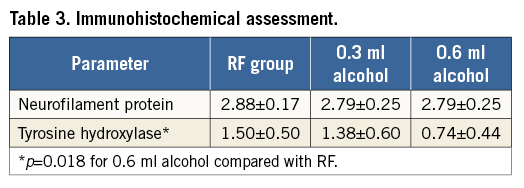
Immunostaining of NFP for the recognition of axons within nerve fascicles ranged from 1 to 3 (weak to strong) across all treatment groups. Mean NFP scores were comparable among the three groups (p=0.70). TH is an efferent nerve marker. Weak staining or absence of staining suggests a reduction or absence of nerve function. The mean TH scores were 1.5, 1.4, and 0.7 for the RF, 0.3 mL, and 0.6 mL group, respectively. The mean TH score was significantly lower in the 0.6 mL alcohol group compared to the RF group by post hoc analysis (p=0.018) (Table 3). There was a discrepancy noted between scoring by histopathological morphology and scoring by immunostaining. Many nerves demonstrating minimal to absent injury on histology showed only a weak reaction to TH. This discrepancy suggests that nerves damaged by any of the three treatments may recover morphologically in three months, but may not recover functionally in three months. Histopathology of all kidneys was within normal limits.
Discussion
Several findings are important. First, tissue injury (ablation) extended significantly deeper, and ablation involved a greater area and occurred in a more circumferential manner with alcohol-induced chemical neurolysis than with radiofrequency ablation. Second, there was a significant reduction in RTNEC with renal denervation compared to historical controls, regardless of the device used and a strong trend towards lower RTNECs with alcohol-induced chemical neurolysis compared with RF ablation. Third, based on the observed trends of tissue injury depth and area and RTNEC, it appears that chemical neurolysis with 0.6 mL might be more effective than with 0.3 mL or single-electrode RF ablation. Finally, renal denervation by chemical neurolysis via percutaneous alcohol infusion was safe.
Data describing the effect of renal denervation using the single-electrode RF Symplicity catheter in an animal model are limited to a qualitative description of renal nerve injury4-6. However, data systematically describing the effect of renal denervation on the extent of renal nerve injury (i.e., tissue injury depth and area) using the single-electrode RF Symplicity catheter have not been published. Moreover, to our knowledge, prospective, randomised, blinded comparisons of histological and physiological effects of different denervation methods have not been performed.
While a relationship between completeness of renal denervation and a reduction in sympathetic drive or blood pressure has not been proven, it is likely that a more complete denervation will more effectively lower sympathetic nerve activity, and blood pressure. Hence, more complete denervation (depth, circumference and area), as was observed with chemical neurolysis in this study, could potentially translate into a more consistent and larger effect upon blood pressure lowering.
In human histologic studies, using pressure-fixed arteries, renal nerves are typically located within 10 mm of the renal artery lumen. In a human autopsy study (n=20), 50% and 25% of nerves were located at a distance greater than 2.44 mm and 4.28 mm from the lumen, respectively7. Likewise, in a porcine model, only 52% of renal sympathetic nerves were located within the first 2.5 mm of tissue depth8. Hence, to achieve as complete a denervation as possible, renal nerve injury to tissue depth beyond 2-4 mm from the lumen might be desirable.
Importantly, despite widespread use and study in clinical trials, to date there are no published reports systematically describing renal nerve injury depth using single-electrode RF energy. However, RF-induced tissue injury depth (in cardiac or skeletal muscle) is dependent on the energy intensity and the force of electrode contact applied9. To our knowledge, the only peer-reviewed published report characterising renal nerve injury depth after RDN is an autopsy report of a woman who died 12 days after undergoing RDN with a single RF energy electrode10. Nerve injury was limited to a 2 mm distance from the intimal surface and was accompanied by wedge-shaped transmural damage to the renal artery wall, with a broad base at the intima.
It is, therefore, not surprising that in previously presented animal studies there was only a modest (47%) reduction in renal tissue norepinephrine concentration after renal denervation with the single-electrode RF Symplicity catheter (four ablations per main artery only)11. This may be due to a limited renal injury depth or to variable success at reaching all quadrants of the artery, or both, with a single-electrode RF denervation catheter. Multi-electrode catheters and strategies of ablating main and side branches may narrow the variability in efficacy as measured by RTNEC11. However, it is not likely that endovascular renal denervation with any energy source without cooling of the interface between the electrode and inner vessel surface would allow renal nerve injury beyond 2-4 mm without substantial thermal injury to the intima and media. Cellular swelling and coagulation of connective tissue within the media and adventitia have been shown after catheter-based radiofrequency renal denervation in a porcine model4. In humans, examination of the arterial wall with optical coherence tomography immediately after single- or multi-electrode radiofrequency denervation shows endothelial-intimal oedema and local thrombus formation in the majority of renal arteries12,13. Though longer-term consequences of such changes may be uncommon, at six-month follow-up after radiofrequency denervation in an animal model, fibrosis of the deep media and underlying adventitia, external elastic lamina disruption and intimal thickening at the denervation site occur5. In humans, longer-term histological follow-up after denervation does not exist for obvious reasons. As a consequence of vascular injury, renal artery stenoses have been reported after RF as well as ultrasound energy application14-16. In an attempt to maintain vascular integrity and patient safety, energy levels delivered, while providing renal nerve injury up to tissue depths of 2-4 mm, may not allow deep enough nerve injury to provide complete denervation and optimal efficacy.
The achievement of complete renal sympathetic denervation may not only require injury to deep enough tissue levels but also injury distribution in a circumferential manner. Single RF energy delivery systems may have shortcomings, as circumferential orientation of the electrode tip is difficult without three-dimensional feedback of the catheter tip location. To illustrate tissue injury with a single-electrode RF catheter: in a typical renal artery with luminal diameter of 6 mm, the perimeter at a distance of 2 mm from the lumen-to-intima interface is 31 mm. In the optimal scenario, assuming that the RF electrode will lead to a 2 mm diameter injury at a depth of 2 mm from the lumen-to-intima interface, four RF applications (the average number in the SYMPLICITY HTN-3 trial) will only lead to injury of 8/31 (26%) of the renal artery circumference.
RDN by chemical neurolysis via percutaneous perivascular alcohol infusion with the Peregrine System has the advantage of reaching deeper nerve fibres (mean nerve injury depth of 3.9 mm with RF ablation versus 6.6 mm and 8.2 mm with chemical neurolysis using 0.3 mL and 0.6 mL of alcohol) and affecting a greater tissue ablation area (11.0 mm2 with RF ablation versus 30.8 mm2 and 41.6 mm2 with chemical neurolysis using 0.3 mL and 0.6 mL of alcohol). Moreover, tissue injury is distributed in a circumferential manner in the overwhelming majority of arteries treated with either dose of alcohol, whereas it is not circumferential in any of the arteries treated with RF energy using single-electrode RF ablation (Figure 5). In further support of the latter finding, circumferential distribution of dye injected using the Peregrine System has also been shown at necropsy immediately after infusion2. Deeper tissue injury involving a greater area with more circumferential distribution may explain a trend towards a more pronounced reduction of RTNEC after chemical neurolysis compared with the single-electrode RF catheter in our study (83% and 78% with 0.6 mL and 0.3 mL of alcohol versus 66% with RF ablation). Of note, the reduction in RTNEC was similar to the reduction in NE concentration reported previously at two weeks after denervation with alcohol-induced chemical neurolysis using the same device as in this study (78% using 0.3 mL and 88% using 0.6 mL)2.
Despite the operator’s significant experience with single-electrode RF ablation using the Symplicity catheter, there was a shorter procedural time with the Peregrine System, in the absence of prior operator experience with this device. This suggests that success with the Peregrine System will be simpler to achieve, and should not be operator-dependent. It is also likely that the shorter procedure times would translate into less radiation exposure for both patient and staff.
Limitations
Several limitations apply. First, though all investigators performing follow-up examinations were blinded, bias on the part of the independent pathologist examining histological sections is possible because, by virtue of experience examining tissue specimens of renal arteries after single-electrode RF ablation as part of other studies performed by the independent laboratory, it may have been apparent that some tissue sections were more consistent with single catheter radiofrequency ablation than alcohol infusion. Second, as previously described, the randomisation process was modified to allow randomisation between RF ablation and chemical neurolysis in animals with main renal arteries ≥20 mm in length. Those with a shorter main renal artery were randomised to 0.3 ml or 0.6 ml of alcohol infusion. Thus, it is conceivable that the more favourable results achieved with alcohol infusion were related to a shorter renal artery length. In this context, it is important to mention that the mean renal artery lengths did not differ between the treatment groups, with the exception of the left renal artery in the 0.6 ml alcohol infusion group. Third, though evidence to suggest that the number of ablations performed per renal artery may be associated with procedural success or magnitude of blood pressure reduction is limited17, it is possible that a more pronounced RTNEC reduction or greater area of ablation by morphometric analysis may have been achieved with a greater number of ablations per artery or by ablation in renal artery branches. Fourth, in our study, pigs that underwent radiofrequency renal denervation were treated with a first-generation radiofrequency catheter. While some data suggest that second-generation radiofrequency catheters may be more effective than first-generation single-electrode catheters, superior efficacy and safety has yet to be demonstrated in a randomised trial. Fifth, though both RTNECs of control animals and treated animals were measured in the same facility using identical methods by blinded laboratory staff, measurements were made at different time points and, potentially, by different laboratory staff. Sixth, RTNECs were used as a surrogate for the activity of the renal sympathetic nervous system activity. Though physiologically plausible, it has not been proven that RTNECs correlate with the activity of the renal sympathetic nervous system assessed by other methods such as norepinephrine spillover measurements. Seventh, though no adverse events occurred and there was no evidence of injury to the renal arteries or surrounding tissue up to three months, longer-term (beyond three months) safety cannot be assured. Eighth, true RTNEC baseline levels are unknown. Finally, the number of animals treated was limited, thereby affecting the strength of any statistical calculations.
Conclusions
The effect of percutaneous renal denervation on the renal artery is device-dependent. Based upon the parameters evaluated in this animal model, alcohol-mediated chemical neurolysis using the Peregrine System is safe and appears more effective than single-electrode radiofrequency ablation using the Symplicity catheter. In this context, differences in reported blood pressure-lowering effects in clinical trials may be related to differences in device design. Superior efficacy and shorter procedural times with the Peregrine System were achieved without any prior operator experience, suggesting ease of use and procedural efficacy that are largely independent of operator experience.
| Impact on daily practice Studies of renal denervation for the treatment of hypertension have been accompanied by variable results. Hence, the efficacy of this therapy remains controversial. It is conceivable that not all devices lead to equivalent results. To date, the histological and biochemical results of renal denervation using different devices or concepts have not been studied in an organised fashion. Importantly, our results suggest that efficacy is device-dependent. Therefore, successes or failures to achieve blood pressure reduction in the past and future may, at least in part, be related to the technology used. Our findings support the need for human clinical trials to assess whether the favourable histological and biochemical effects of alcohol-mediated chemical neurolysis in an animal model cause meaningful blood pressure reductions in humans. |
Conflict of interest statement
S. Bertog, H. Sievert and L. Vaskelyte’s institution has ownership interest in or has received consulting fees, travel expenses or study honoraria from the following companies: Abbott, Ablative Solutions Inc., Access Closure, AGA, Angiomed, Arstasis, Atritech, Atrium, Avinger, Bard, Boston Scientific, BridgePoint, Cardiac Dimensions, CardioKinetix, CardioMEMS, Coherex, Contego, CSI, EndoCross, EndoTex, Epitek, Evalve, ev3, FlowCardia, Gore, Guidant, Guided Delivery Systems, Inc., InSeal Medical, Lumen Biomedical, HLT, Kensey Nash, Kyoto Medical, Lifetech, Lutonix, Medinol, Medtronic, NDC, NMT, OAS, Occlutech, Osprey, Ovalis, Pathway, PendraCare, Percardia, pfm medical, Rox Medical, Sadra, SJM, Sorin, Spectranetics, SquareOne, Trireme, Trivascular, Velocimed, Veryan. T. Fischell is the owner of Ablative Solutions Inc., the company sponsoring this study and providing the devices used. V. Ghazarossian, F. Vega, E. Ladich, K. Yahagi, R. Virmani and D. Kent have received consulting fees from Ablative Solutions Inc. A. Pathak has provided consulting services to Ablative Solutions Inc.

