
Abstract
Aims: We report the use of a novel endovascular approach using chemical neurolysis, via periadventitial injection of dehydrated ethanol (EtOH) to perform renal artery denervation.
Methods and results: A novel, three-needle delivery device was introduced into the renal arteries of adult swine using fluoroscopic guidance. EtOH was injected bilaterally with one injection per artery, via the three needles into the adventitial and periadventitial space, using EtOH doses 0.15 ml/artery; n=3, 0.30 ml/artery; n=3, and 0.60 ml/artery; n=3, with saline injection as a sham control (0.4 ml/artery; n=3), and naive subjects (n=7) as a true negative control. The renal parenchymal norepinephrine (NE) concentration at two-week follow-up was the primary efficacy endpoint. The mean renal NE reduction was 54%, 78% and 88% at doses of 0.15 ml, 0.30 ml and 0.60 ml, respectively (p<0.0001 vs. controls). Histological examination revealed marked, and deep, circumferential renal nerve injury at depths of 2-8 mm from the intimal surface. There was no evidence of device-related or EtOH-induced injury to the intimal layers. In some samples at the higher EtOH doses, there was focal loss of smooth muscle cells in the outer media. Angiography at 45 days demonstrated normal appearing renal arteries with no detectable stenoses (n=8).
Conclusions: Circumferential adventitial delivery of very low doses of EtOH may be a promising alternative to energy-based systems to achieve dose-dependent, and predictable renal denervation. Further study is warranted.
Introduction
From the 1930s to the 1950s surgical renal sympathectomy was used to treat severe hypertension1-3. Despite the successful lowering of blood pressure (BP) observed with surgical denervation, this technique was abandoned due to a relatively high morbidity and mortality, and as a result of the development of more effective oral antihypertensive medications.
Recently, catheter-based renal sympathetic denervation has been performed using a point-by-point, monopolar radiofrequency (RF) ablation catheter from within the renal artery4-11. This technique has been shown to disrupt renal sympathetic nerve activity4-7, resulting in substantial and sustained blood pressure lowering in patients with severe and medically resistant hypertension4-11.
The ability to lower blood pressure using a catheter-based ablative technique from within the renal artery has led to a proliferation of new technologies intended to expand and validate this observation, and to improve the ease-of-use, safety and predictability of catheter-based renal sympathetic denervation12-14.
Virtually all of these new denervation devices have focused upon “energy-based” denervation, utilising predominantly RF or ultrasound catheters. These catheters are designed to deliver ablative thermal injury through the intima and medial layers of the renal artery, in order to target and destroy the sympathetic nerve fibres that traverse from the aorta to the kidney within the adventitial and periadventitial space surrounding the renal arteries4-12.
A number of these “next generation” RF and ultrasound transmural thermal ablation catheters have been tested in patients and have validated the results from the original Symplicity trials, with significant BP lowering seen after renal sympathetic (thermal) denervation11-14.
Despite the moderate efficacy of both the first and next generation RF and ultrasound catheters, there are limitations and potential safety concerns associated with the use of transmural thermal injury traversing the intimal and medial layers of the renal artery in order to create thermal injury to the sympathetic nerves that may run from 2-10 mm deep from the intimal surface15-18.
Given the potential limitations of RF and ultrasound, we have evaluated the use of chemical renal denervation, using very small volumes of ethanol (EtOH), a known potent neurolytic agent, delivered precisely and locally to the adventitial space of the renal artery as a means of performing renal sympathetic denervation. The purpose of this study was to evaluate the safety and efficacy of chemical neurolysis, via adventitial injection of very small doses of dehydrated EtOH, as a means to perform safe, efficient and effective renal artery sympathetic denervation in a porcine model.
Methods
A novel, three-needle delivery device (Peregrine System™; Ablative Solutions, Inc., Kalamazoo, MI, USA) was introduced via the femoral artery into renal arteries of adult swine using fluoroscopic guidance. The drug injection catheter is an endovascular delivery catheter that contains three distal needles housed inside individual guide tubes, which are contained within the catheter. The catheter has a steerable, radiopaque 2 cm fixed, floppy guidewire at its distal end to minimise renal artery trauma and allow steerability, when needed, into appropriate branch vessels (Figure 1, Figure 2A).
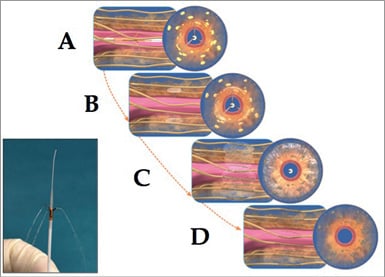
Figure 1. Schematic drawings showing sequence of chemical denervation with EtOH. A) The guide tubes and injection needles have been deployed in the renal artery, such that the tips of the three needles are at ~120 degree separation, and have penetrated to a depth of ~3.5 mm relative to the intimal surface. Yellow structures represent renal sympathetic nerves. B) The 98% dehydrated EtOH is injected into the adventitial and periadventitial space, ablating the nerve fibres (yellow). C) The EtOH has spread 360 degrees, creating circumferential nerve injury out to a depth of ~8 mm from the intimal surface. D) shows the effects observed at two weeks after “chemical denervation”, with essentially complete, circumferential, segmental nerve ablation. The inset image (lower left) shows the distal end of the actual Peregrine System™ with a small injection of fluid emanating from the three needle tips
This study was conducted under the general principles of Good Laboratory Practice (GLP) regulations as set forth in 21 CFR 58. The protocol for this study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the test facility, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Animals were received, examined, quarantined and housed as per standard operating procedures. Animals were pre-medicated with 325 mg of aspirin and 75 mg of clopidogrel by mouth once daily for two days before the procedure. The animals were assigned to study groups at random before the procedure began.
The animals were pre-medicated with intramuscular injection of telazol combined with atropine. When recumbent, animals were anaesthetised with a mixture of isoflurane and oxygen delivered via a facemask. When sufficiently anaesthetised the animals were intubated and connected to a closed-circuit anaesthesia system and maintained on isoflurane combined with oxygen. Blood was collected for evaluation of haematology: complete blood count (CBC) and serum chemistry. Urine was obtained via cystocentesis.
After the animals had been prepared for sterile surgery one femoral artery was accessed using the Seldinger technique, and a 7 Fr introducer sheath was put in place. Intravenous heparin was given to all animals to achieve an ACT of >250 sec. In all cases the right and left renal arteries of the pig were engaged using a 7 Fr renal double curve (RDC) guiding catheter. Prior to ethanol or saline injections, angiography of each renal artery was performed using iodixanol contrast diluted by 25% with normal saline.
The Peregrine™ injection catheter is intended to be used under fluoroscopic guidance by a single operator using standard endovascular techniques and to allow safe and reproducible fluid injection into the adventitial and periadventitial space of a target vessel (Figure 1, Figure 2). The needles of the Peregrine catheter are within the guide tubes, which are housed inside the body of the catheter. Once deployed, the three tubes serve to “centre” the device in a reproducible manner within the renal artery. These atraumatic tubes have radiopaque distal tip markers so that one can clearly define the position of the tubes, particularly when contrast is injected via the guiding catheter (Figure 2A).
The 0.008” needles which are housed within the distal tip of the tubes are advanced to a depth of 3.5±0.25 mm deep in the intima (i.e., beyond the tip of the guide tube). The specialised handle allows simultaneous advancement of the three injection needles. These tiny needles are radiopaque so that they can easily be seen under fluoroscopy. The injectant, in this case ethanol, is delivered through a Luer lock connector at the proximal end of the handle, resulting in the infusion of the ethanol through the tips of the needles of the Peregrine catheter, and directly into the adventitial and periadventitial space.
Thus, the device is advanced into the left or right renal artery via the guiding catheter. Once the operational section of the device is positioned within the target site in the mid portion of the renal artery, the three guide tubes are simultaneously deployed against the intimal surface, using the advancement mechanism in the control handle (Figure 1).
It should be noted that these needles are the equivalent of a ~30 gauge needle so that they can be safely advanced through the renal arterial wall without causing bleeding. Prior to conducting this study we confirmed that needles of this size could be repeatedly advanced through the wall of the renal artery of pigs that had been pre-treated with high doses of heparin (ACTs 300-600), with no detectable bleeding at the needle puncture sites. This is a key observation relevant to the safety of this approach.
Once the tubes and needles are deployed, it is easy to confirm, fluoroscopically, that the needle tips are well outside the luminal space and ~2.5-3.0 mm deep to the media and the external elastic lamina in a normal porcine renal artery (Figure 2A). This corresponds to an injection depth that approximates the border between the renal artery adventitia and periadventitia, and which corresponds to a depth to the middle of the renal sympathetic nerve field, as defined in pressure-fixed human histopathological studies by Virmani et al18.
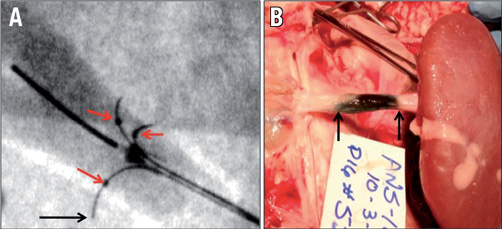
Figure 2. Evidence of circumferential spread of fluid with three-needle delivery system. In (A), the device is deployed with the three radiopaque needles spread at ~120 degrees, one to another. The lumen of the renal artery is faintly opacified with dilute contrast so that one can appreciate the depth of needle tip penetration (arrow) relative to the intimal surface. The radiopaque tips of the guide tubes are indicated by the red arrows. In (B), 0.125 ml of EtOH admixed with 0.25 ml of methylene blue was injected via the three needles of the catheter. The photo shows the renal artery with most of the surrounding tissue removed, leaving only adventitial tissue immediately surrounding the vessel. This demonstrates circumferential spread of the EtOH/methylene blue in the adventitial space at one hour after 0.15 ml total injection volume. The arrows indicate the margins of the treatment site.
The successful deployment of the tubes and needles is confirmed by angiography. The EtOH or saline (sham) fluid is then administered, using a 1.0 ml Luer lock syringe attached to the proximal injection lumen at the handle of the catheter. This injection lumen is in fluid continuity with the distal end of all three needles. The injection is performed over 20-30 seconds.
Three volumes of EtOH were used in this study: 0.15 ml/artery (n=3 pigs/6 arteries), 0.30 ml/artery (n=3 pigs/6 arteries) and 0.60 ml/artery (n=3 pigs/6 arteries). A procedural control group was also studied using the injection of 0.4 ml of saline/artery (n=3). This was a “sham” arm to control for non-specific effects that might be caused by mechanical injury from either the guide tubes or the needles, and/or any non-specific effects of fluid delivery. Once the treatment agent was injected, the dead space of the catheter was flushed with a very small volume of normal saline. After treatment of the first renal artery the device was removed from the animal, inspected and flushed. The contralateral renal artery was then engaged and the same fluid injection sequence was performed in the contralateral renal artery. After the treatment of the second renal artery, the animals were recovered and housed for restudy and sacrifice at two weeks post intervention. The animals were treated with aspirin 162 mg/day for seven days after intervention.
The circumferential spread of EtOH was evaluated in separate experiments by combining 0.125 ml of EtOH with 0.25 ml of methylene blue (stain). The volume of 0.15 ml was then injected under fluoroscopic guidance. Immediate necropsy was performed and demonstrated reproducible and circumferential spread of the 0.15 ml of the EtOH/methylene blue mixture (Figure 2B). Histopathology was also used to evaluate circumferential spread of alcohol by having the pathologist evaluate and document the location (in terms of circumference) of any noted neuritis and neurolysis. The histopathological examination showed extensive and circumferential nerve injury at the 0.15, 0.30 and 0.60 ml EtOH injection volumes.
The safety and effectiveness of the device were evaluated. The efficacy of denervation was determined by measurement of renal parenchymal norepinephrine (NE) levels (analysed by high performance liquid chromatography [HPLC]), with electrochemical detection), and using histopathologic evaluation of the perirenal nerves at the end of the two-week survival period. Safety was evaluated by histopathologic evaluation of the renal artery and kidney as well as by evaluation of clinical pathology. Blood and urine were collected in all animals treated with the device for evaluation of systemic and renal health at baseline and at the time of sacrifice.
The animals were kept alive for 14±3 days after treatment. At the end of the study period the animals were anaesthetised, and angiography of the treated right and left renal arteries was obtained to evaluate vessel patency and to look for any luminal narrowing compared to baseline angiography.
After angiographic follow-up at 14 days, a necropsy was performed. The renal arteries and kidneys were harvested for histopathological evaluation. Gross pathology to examine the status of the renal arteries was performed to look for renal artery abnormalities such as aneurysms, perforations, dissections, haematoma, etc., as well as inspection of the surrounding tissues for any abnormalities. The renal arteries were gently flushed with saline and then perfused with 10% neutral buffered formalin. The renal arteries and kidneys were harvested, retaining the periadventitial tissue around the artery. The renal artery tissue was embedded in paraffin using standard techniques. Tissue was stained with haematoxylin and eosin stain (H&E). Multiple sites from each renal artery segment were labelled and sent for (blinded) microscopic evaluation by a board-certified veterinary pathologist.
Four samples were obtained from random locations at each of the proximal, mid and distal regions of each kidney for a total of 12 samples/kidney. The tissue samples were weighed, placed in cryovials and flashfrozen by immersion in dry ice. The frozen samples were then stored at –70°C. They were sent in dry ice to an independent laboratory for (blinded) measurement of renal parenchymal norepinephrine levels.
Renal norepinephrine concentrations in the treated animals from this study were also compared to values from naive control animals of the same age and species (n=7) with renal tissue sampling performed in an identical fashion to the treated animals. The safety of ethanol injection was also assessed in a separate nephrotoxicology study (n=4). After deep engagement of the renal arteries, 0.6 ml of EtOH was injected directly in both the right and left renal arteries (1.2 ml total EtOH/animal), over 20-30 seconds to replicate the timing of injection into the adventitial space when therapeutic EtOH neurolysis was performed. These animals had serial measurement of serum, blood urea nitrogen (BUN), creatinine, electrolytes and body weight at days 1, 7 and 30 after the injection. Histopathological evaluation of the renal parenchyma was performed in all such treated kidneys at 30 days to look for any evidence of renal injury.
Additional longer-term safety evaluation was obtained with angiographic follow-up at 45 days, in four additional animals (n=8 arteries) treated with 0.3 ml EtOH. These studies were performed to look for any evidence of late renal artery stenosis.
For statistical analysis, between-group comparisons were made using a Wilcoxon rank-sum test, performed using R, version 2.14.1 (R Foundation for Statistical Computing, Vienna, Austria). Data are shown in graphs as mean ± SD. A p-value of <0.05 was considered significant.
Results
Device success was defined as successful injection of the designated fluid without serious adverse events. The device was used successfully in all 12 animals and 24 renal arteries. Procedure time, measured from the advancement of the device into the renal artery, followed by deployment of needles, injection, and withdrawal into the guiding catheter averaged approximately 90 seconds for each renal artery (range: 55-140 seconds). A small haematoma at the femoral access site was recorded in one animal. There was no other study-related morbidity or mortality.
Measurements of renal tissue NE showed an essentially linear dose response (R2=0.95) between the EtOH volume delivered and the reduction of the renal parenchymal NE level (Figure 3, Figure 4). The mean renal NE reductions were 54%, 78% and 88% at doses of 0.15 ml/artery, 0.30 ml/artery and 0.60 ml/artery, respectively (p<0.0001 vs. combined controls; Figure 3 and Figure 4). The other statistical comparisons are shown in Figure 3 and demonstrate a statistically significant reduction (p<0.05) in renal parenchymal NE at all three doses vs. sham controls.
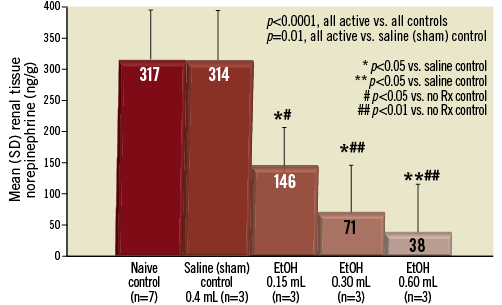
Figure 3. Bar graph showing the dose-response effect of adventitial EtOH delivery upon the renal parenchymal norepinephrine level at 14±3 days. There is a marked and dose-dependent reduction of NE levels versus both naive control animals and sham control animals injected with saline. Mean NE reduction was 54% with 0.15 ml, 78% with 0.30 ml, and 88% using 0.60 ml/artery, respectively. Standard deviation (SD) for each data set as shown. p-values as shown.
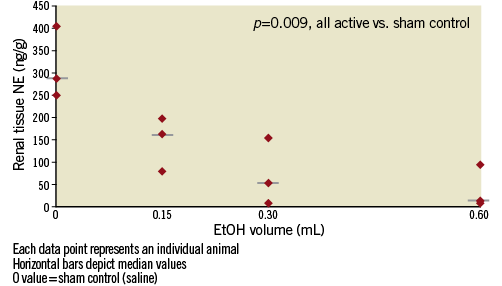
Figure 4. Graph showing the dose-response effect of adventitial EtOH delivery upon renal parenchymal norepinephrine level at 14±3 days. Each data point represents an individual animal. Horizontal bars show median values. The “0” dose is the saline (sham) control group. There is a marked and dose-dependent reduction of NE (p=0.009 EtOH-treated vessels vs. controls).
Angiographic follow-up of all 24 treated vessels at 14±3 days showed no evidence of renal artery narrowing at EtOH doses of 0.15, 0.30 or 0.60 ml/artery. No aneurysmal changes, thrombus or other abnormalities were noted. Angiography at the 45-day time point in four additional animals (eight arteries) treated with 0.3 ml EtOH demonstrated no detectable renal artery narrowing (Figure 5).
Histological examination revealed marked, and deep, circumferential renal nerve injury at depths of 2-8 mm from the intimal surface (Figure 6, Figure 7A). There was no discernible nerve injury in the saline, sham-control injected animals (Figure 7B). Nerve injury in the EtOH treated vessels was characterised by vacuolisation, loss of internal architecture, and the development of perineural fibrosis (Figure 6, Figure 7A).
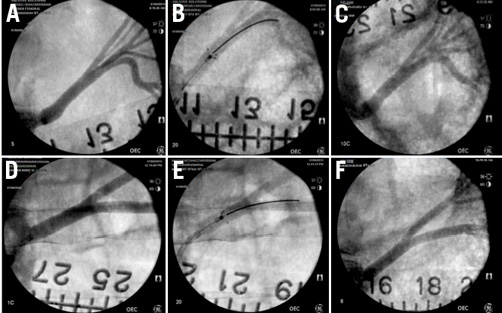
Figure 5. Angiography of renal arteries at time of treatment, during deployment of the Peregrine System™, and at 45-day follow-up. (A) and (D) show the pre-treatment renal arteries. (B) and (E) show the angiographic image of the Peregrine System™ at the time of injection. (C) and (F) show 45-day angiographic findings in these two animals treated with 0.30 ml EtOH. There is no detectable luminal narrowing or other abnormality. These images are representative of all eight arteries studied.
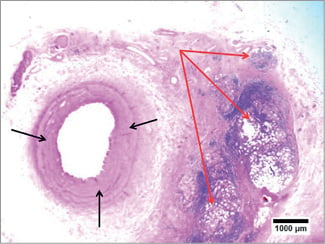
Figure 6. Histopathology (H&E) of renal denervation at 14 days with 0.15 ml EtOH injection. The renal artery (intima and media) appears intact without evidence of injury or inflammation (black arrows). The reaction to the EtOH appears quite limited to the adventitial layers. There is severe damage to the deep renal nerve bundles with vacuolisation, nerve fibre disruption, inflammation and fibrosis of the perineural structures at a depth of 4-8 mm deep to the intima (red arrows).
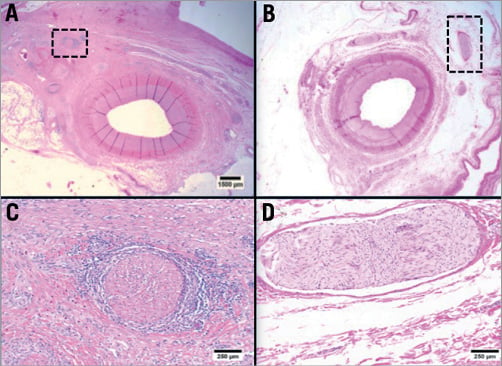
Figure 7. Histopathology (H&E) of renal denervation at 14 days with 0.30 ml EtOH injection (A and C) versus saline control injection (B and D). The renal arteries (intima and media) appear intact with no evidence of injury or inflammation (A and C). In contrast, there is marked destruction of a deep renal nerve bundle with vacuolisation, nerve fibre disruption and inflammation of the perineural structures at ~5 mm deep to the intima (black hatched box in a and high powered micrograph in B). Unlike the nerve bundle of the EtOH-treated artery (B), the nerve fibre bundles in the saline-treated (sham) control appear totally intact (D).
There was no evidence of device-related or EtOH-induced injury to the intimal layer of treated vessels. There were no thrombi, dissections, aneurysms, perforations, haematomas or other device-related pathologies. There was no evidence of EtOH-induced injury to the intimal or medial layers of the renal arteries at the 0.15 ml dose.
At the higher doses (0.30 and 0.60 ml EtOH) there was occasional, focal pallor of some smooth muscle cells in the outermost media, typically originating at the adventitial surface and associated with injection sites. At some of these sites there was focal proteoglycan deposition. Inflammatory responses were typically absent from the media, and the vessels appeared to be healing normally. There was no discernible injury to tissue deep in the periadventitial plane.
There were no adverse nephrotoxic or systemic effects seen. The pigs’ serum creatinine, BUN and electrolytes remained unchanged over the study period. Finally, direct injection of EtOH into the renal artery, at 200% the likely therapeutic dose (i.e., 0.60 ml vs. 0.30 ml), resulted in no detectable renal toxicity as measured by creatinine, BUN, or electrolytes measured at 1, 3, 7 and 30 days after EtOH injection (all p’s = NS). There were no discernible renal pathological effects seen on sectioning of the renal parenchyma at 30-day follow-up.
Discussion
In this study we have demonstrated the apparent safety and predictability, as well as the dose-dependent efficacy of low-dose ethanol injection via microneedles into the perivascular space to achieve circumferential sympathetic nerve ablation (PeriVascular Renal Denervation, or PVRD™), with minimal injury to the normal renal artery intimal and medial layers. The ability to target and deliver a neurolytic agent to a deep periadventitial space may allow more complete renal denervation than might be easily or safely obtained using energy-based systems from within the renal artery.
Renal denervation may prove to be a valuable intervention to treat severe hypertension as well as a number of other conditions that may be driven by sympathetic imbalance or “overdrive.” Denervation of the renal artery has been shown to be effective in the treatment of refractory and drug-resistant hypertension4-7, and possibly in the treatment of congestive heart failure19, central obstructive sleep apnoea20,21, left ventricular hypertrophy22, metabolic syndrome23,24, chronic kidney disease25,26 and in patients with atrial and/or ventricular arrhythmias27,28. There is a need to develop and evaluate the safest and most effective methods to perform renal denervation.
The drug-delivery catheter used in this study is a novel endovascular delivery device that contains three distal needles housed inside individual guide tubes, which are contained within the catheter. The catheter is used under fluoroscopic guidance by a single operator using standard endovascular techniques to access the vessel of choice and perform injection into the adventitial and periadventitial space of that vessel. The radiopaque microneedles are deployed with minimal trauma to the normal renal arterial wall and to target delivery in the adventitial and periadventitial space. There is no obstruction of renal blood flow during the deployment of this device. The device is very simple to use, with catheter positioning and denervation being performed in less than two minutes from the time that the catheter enters the renal artery via the guiding catheter in virtually all cases. There were no device-related complications.
The known neurolytic agent, Dehydrated Alcohol Injection, USP (ethanol - EtOH, 98%), was chosen for the evaluation of the drug-delivery catheter used in this study29-33. Ethanol is indicated and FDA approved for therapeutic neurolysis, and produces injury to tissue cells by dehydration and by precipitation of protoplasm. At very low doses, ethanol is known to produce neuritis and nerve degeneration (neurolysis). Deliberate injury to nerves by the targeted injection of ethanol results in more or less enduring block of sensory, motor and autonomic function28-32.
As seen in this study, the dose of ethanol that is effective in creating nearly complete renal sympathetic neurolysis is a dose that is so small that it will produce no apparent systemic effects. Even when double the expected therapeutic dose of 0.30 ml EtOH was purposely injected directly into the renal artery, we could not detect any signs of renal toxicity. Even the highest dose used in this study (0.60 ml) contained less alcohol than a single alcoholic drink.
The porcine renal denervation model has been well characterised, and does appear to predict efficacy in the treatment of refractory hypertension in human subjects. In this study it was possible to verify and quantify denervation of the kidneys by measurement of the renal parenchymal levels of the neurohormone norepinephrine (NE). We further validated the neurohormonal effects of ethanol-mediated denervation by using careful and blinded histological examination of both treated and sham-controlled arteries.
The histopathology demonstrated profound and circumferential renal sympathetic nerve damage, with nearly complete sparing of the normal intimal and medial architecture of the renal artery, using doses of 0.15-0.60 ml of EtOH/artery. Evaluation at this time point indicated normal arterial healing, although evaluation at longer time points is needed to evaluate safety more fully.
At ethanol doses of 0.30 and 0.60 ml there was essentially complete denervation, as judged by the 78% and 88% reduction in renal parenchymal norepinephrine levels, respectively. These reductions observed with 0.30 and 0.60 ml EtOH are substantially equivalent to the reductions seen with surgical denervation in a porcine model.
Despite the reasonable efficacy of both the first and next-generation RF and ultrasound catheters3-7, there are a number of potential limitations and safety concerns associated with the use of transmural thermal injury using either RF or ultrasound, traversing the intimal and medial layers of the renal artery. In order to create thermal injury to the sympathetic nerves that may run from 2-10 mm deep from the intimal surface15-18, there will be collateral damage to the intimal and medial layers of the renal artery wall. The ability to damage the renal sympathetic nerves predictably in a dose-dependent fashion, using very low volumes of EtOH delivered in the adventitial space, may have a number of potential advantages over energy-based systems.
The concerns and limitations of transmural thermal-injury catheters, that could potentially be overcome with chemical neurolysis, include: 1) potential failure to denervate deeper nerve fibres adequately due to the heat sink as the thermal injury traverses the renal artery wall, resulting in “non-responders” and/or an inadequate BP lowering response4,6,7,9; 2) failure to create circumferential nerve ablation such that many nerves pass uninjured to the kidney, resulting in suboptimal efficacy4,6,7,34; 3) intense pain related to thermal ablation requiring high doses of benzodiazepines in combination with very high doses of narcotic analgesia, with attendant respiratory depressive effects and the risk of prolonging hospitalisation4-10; 4) the risk of luminal thrombus formation on the endoluminal surface at sites of thermal “burn” injury, with the subsequent risk of downstream thrombo-embolisation and potential for renal injury15; 5) large volumes of contrast injected directly into the renal arteries bilaterally during repeated positioning of a unipolar electrode RF system, with the potential risk of contrast-induced renal injury; 6) long case duration with high levels of radiation exposure for the patient and the operator4-11; 7) the expense of purchasing and maintaining complex capital equipment required to perform RF and/or ultrasonic ablation; and, finally, 8) the risk of provoking neointimal hyperplasia and/or negative remodelling from transmural thermal arterial injury, which may result in subsequent renal artery stenosis and recurrent hypertension16,17.
Although the limitations of thermal ablation for renal denervation are real, it remains to be determined how effectively chemical denervation with EtOH might overcome each of these drawbacks.
Limitations
There are limitations in any animal model used to try to simulate the safety and/or efficacy of a therapeutic procedure to be used in human subjects. The safety of using microneedles in hypertensive humans will require clinical evaluation.
It should be appreciated that renal parenchymal norepinephrine is only a surrogate marker for efficacy in patients. The exact correlation between drops in renal parenchymal norepinephrine levels and antihypertensive efficacy in humans has not been definitively correlated.
Although these data from this porcine model are encouraging, the true safety and efficacy of ethanol-mediated perivascular renal denervation will need to be validated in longer-term preclinical testing, and eventually in clinical trials.
Summary
In summary, we report the first use of adventitial and periadventitial local delivery of very low doses of dehydrated EtOH to perform successful renal sympathetic denervation. Circumferential and deep periadventitial delivery of very low doses of EtOH has the potential to be a simple, safe, predictable and viable alternative to energy-based systems to achieve substantial and dose-dependent renal denervation, with minimal injury to the normal renal arterial wall.
Acknowledgements
We wish to acknowledge the expert technical contributions of Jeff A. Burke, Phil C. Burke, Andy E. Denison, and Chris S. Hayden from REV•1 Engineering.
Funding
Ablative Solutions, Inc., provided funding for this study.
Conflict of interest statement
T.A. Fischell, D.R. Fischell and V.E. Ghazarossian are principals and co-founders of Ablative Solutions and have equity positions in the company. F. Vega, N. Raju, E.T. Johnson, D.J. Kent and R.R. Ragland have equity positions and/or are paid consultants working with Ablative Solutions, Inc. S.L. Almany is a paid consultant on the Board of Directors of Ablative Solutions, Inc., and a partner in Biostar, LLC, an investor in Ablative Solutions, Inc.

