Abstract
Several position statements provided recommendations regarding the anatomical conditions qualifying for renal denervation catheter application based on the HTN-1 and HTN-2 trials. This manuscript summarises anatomical access artery and renal artery conditions which qualify for catheter-based renal denervation according to the instructions for use and those where treatment is not yet generally recommended, mostly due to limitations of the catheter technologies currently available, e.g., no current device is approved for brachial or radial access.
Introduction
The recommendations regarding access management and selection of suitable renal artery anatomy for catheter-based renal denervation in currently published international position papers1-6 refer to a single renal denervation device, the single electrode radiofrequency Symplicity® catheter (Ardian - Medtronic, Minneapolis, MN, USA). Since 2012, additional radiofrequency or ultrasound based systems received CE mark such as Vessix V2 (Vessix Vascular – Boston Scientific, Natick, MA, USA), OneShotTM (Maya Medical – Covidien, Dublin, Ireland), PARADISETM (ReCor Medical, Ronkonkoma, NY, USA) and EnligHTNTM (St. Jude Medical, St. Paul, MN, USA) with still limited experience in clinical application. Major device limitations of all currently available devices are their relative stiffness due to metallic catheter tip components and their large profile requiring sheath sizes up to 8 Fr or guiding catheter sizes up to 9 Fr. The majority of the devices are wireless systems potentially negatively affecting the navigation of the devices into and through the renal artery.
Suitable anatomical renal artery conditions for percutaneous denervation therapy based on the recent position papers are summarised in Table 11-6.

Percutaneous renal denervation procedure7
STANDARD CASE
PERIPROCEDURAL MEDICATION
Heparin is administered after sheath placement, usually a bolus dose of 5000 IU. ACT should exceed 250 seconds. Regarding pretreatment with antiplatelet drugs no uniform recommendation exists. In patients without an indication for secondary preventive intake of acetyl-salicylic-acid (ASA) we recommend pre-loading with ASA 500 mg the day of the procedure followed by 100 mg per day for at least four weeks8. Dual antiplatelet therapy does not seem to be indicated.
ARTERIAL ACCESS
The femoral access site is currently the only approved approach for percutaneous renal denervation therapy independent of the device used. Side selection, right or left groin, depends on pre-interventional documented access artery abnormalities such as iliac artery tortuosity. The goal is guaranteeing a stable position of the guiding catheter tip at the renal artery origin. The guiding catheter technique is recommended (Figure 1) requiring a 6 to 9 Fr sheath with a haemostatic valve. A standard sheath with a length of 10 or 11 cm is used (various manufacturers, e.g., AvantiTM; J&J, Cordis Corp., Miami, FL, USA, or Terumo Corp., Leuven, Belgium). In tortuous iliac arteries a longer sheath (23 cm, various manufacturers) improves manipulation of catheters and wires. Alternatively, a pre-shaped guiding sheath (Vista Britetip IGTM; J&J, Cordis [Figure 2]; Destination®; Terumo) can be used with limited steerability as compared to guiding catheters. Hereby the device diameter is downsized by 1 Fr (e.g., 5 Fr sheath instead of a 6 Fr guiding catheter) and the risk of access site complications may be reduced.
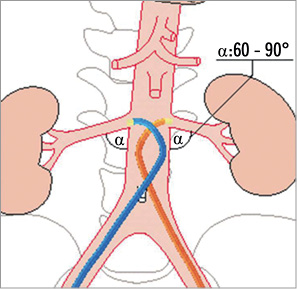
Figure 1. Guiding catheter technique, renal double curve (RDC) configuration.
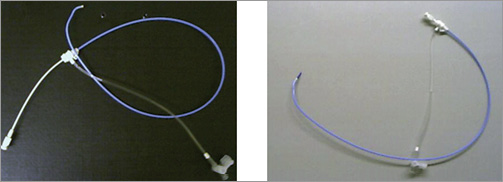
Figure 2. 7 Fr guiding sheath (=5.5 Fr outer diameter) VISTA BRITE TIP® IG (Cordis), RDC configuration, 55 cm in length (also available as 90 cm long sheath in Multipurpose configuration).
BASELINE RENAL ARTERY ANGIOGRAPHY
In an anatomically normal abdominal aorta, the origins of right and left renal arteries are identified best in a 20° left anterior oblique (LAO) projection because the off-take of the right renal artery is slightly anterior (at 10 to 11 o’clock) and of the left renal artery is slightly posterior (at 4 to 5 o’clock, Figure 3). Semi-selective and selective baseline angiography should be performed to determine the optimal projection for the intervention which is in most situations an anterior-posterior projection. Conventional angiography sometimes allows better visualisation of the lesion and the anatomy compared to the digital subtraction technique (DSA) due to fewer movement artefacts. Prior to the first selective renal angiography, the guiding catheter should be cleaned from debris by passive back-bleeding of the catheter or active aspiration of 10 cc of blood via a Y-connector. This technique potentially reduces the risk of renal embolism.
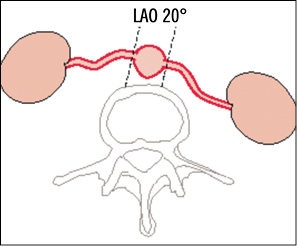
Figure 3. Drawing of the normal anatomy of renal artery orifices in a cross-sectional view (broken line shows optimal c-arm angulation).
TECHNIQUE OF RENAL DENERVATION (SYMPLICITY II CATHETER)
A variety of guiding catheter configurations, such as “renal double curve, RDC”, “hockey stick”, “Amplatz right, AR-1”, “Judkins right, JR-4” or “internal mammarian, IMA” is available to guarantee a stable position at the renal artery origin (Figure 4). The IMA catheter configuration fits in approximately 80 to 90% of the cases. The guiding catheter technique is the fastest technique with the lowest intervention and radiation time. After placing the guiding catheter close to the renal artery origin, nitroglycerine 0.5 mg should be selectively injected into the renal artery and the denervation catheter is then advanced into the distal renal artery main trunk close to the bifurcation into the renal artery branches. Cave: don’t deflect the catheter tip while advancing the device because the tip becomes progressively stiffer when bending the tip! Once the intended position is reached the catheter tip has to be deflected pulling the lever on the handle (Figure 5) and impedance has to be checked. The absolute impedance value indicating a proper vessel wall apposition of the electrode ranges from 220 to 320 Ω and must be stable (±5 Ω) before starting the ablation cycle of 120 seconds.
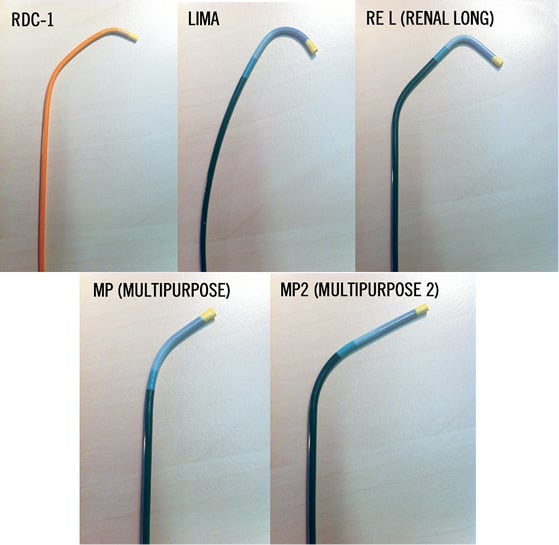
Figure 4. Different guiding catheter configurations available for selective renal artery intubation (standard configuration: LIMA).
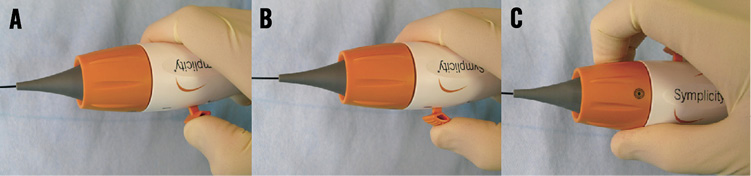
Figure 5. Symplicity II catheter handle features: A) deflect tip by pulling lever towards the handle; B) straighten tip by pushing lever towards front of handle; C) shaft and electrode can rotate independently from handle body, handle rotator has tactile “click” every 45°, dot on rotator gives relative rotational reference.
It is recommended to start the denervation procedure in an inferior orientation of the catheter tip and to perform four to six focal treatments with a distance of ≥5 mm between each location. By pulling and rotating the catheter tip into a new location, an almost circumferential coverage of the renal artery lumen should be enabled. The last radiofrequency ablation cycle should be applied close to the renal artery origin in a superior position (Figure 6). During the entire procedure continuous saline flushing is recommended (Figure 7). Before the catheter is pulled out of the renal artery an angiographic control of the treatment sites should be performed. Thereafter, the catheter should be removed out of the guiding catheter and should be cleaned from thrombus or char. The next step is the treatment of the contralateral renal artery in the same fashion.
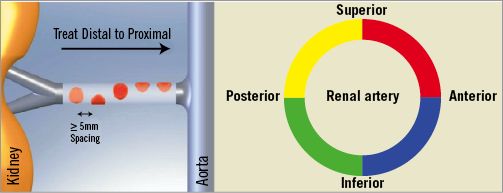
Figure 6. Strategy of renal denervation. Distal: inferior-inferolateral; proximal: superior-superolateral. ≥5 ablations are separated both longitudinally and rotationally (spacing >5 mm) 120 sec each.
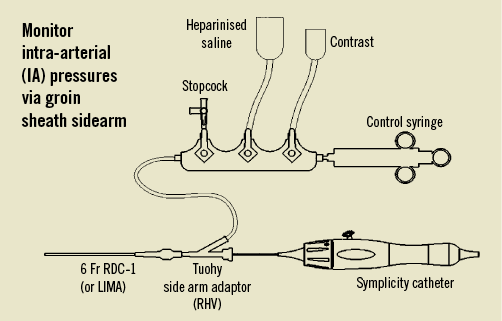
Figure 7. Manifold set-up; contrast should be diluted 50:50.
POST-INTERVENTIONAL RESULT DOCUMENTATION
After the intervention, selective and/or semi-selective angiograms of the renal arteries including the distal branches should be performed to exclude peripheral embolism, dissection or perforation (Figure 8). Intravascular ultrasound or optical coherence tomography is only indicated for study purposes.
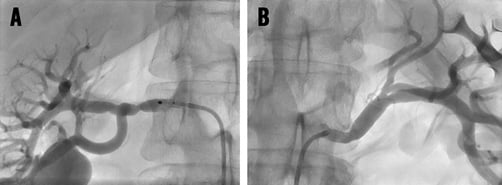
Figure 8. Angiographic control of renal arteries after renal denervation showing mild spasm in a 56-year-old male patient. Note: intrarenal vascular tree incomplete; thus, peripheral embolism cannot be excluded from these images.
COMPLEX ANATOMICAL CASE SCENARIOS
ILIAC ARTERY KINKING
Extensive iliac artery elongation with vessel tortuosity (Figure 9A) can result in kinking of a guiding catheter even when using long sheaths (Figure 9B). Due to the design of the Symplicity II catheter with a floppy tip and a short transition zone to the stiff body of the shaft the catheter can get stuck in such kink zones of the guiding catheter. The introduction of a stiff 0.018 inch guidewire into the guiding catheter crossing the kink zone enables the advancement of the Symplicity catheter due to a slight straightening of the guiding catheter curvature (Figure 9C and Figure 9D).
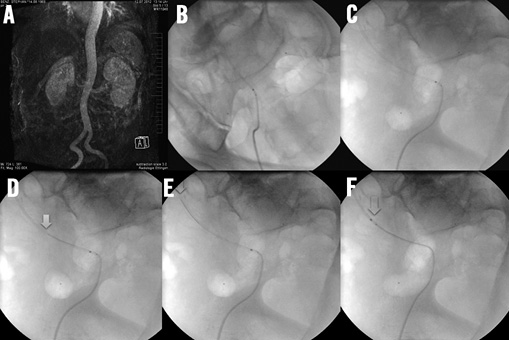
Figure 9. A) Access artery kinking in MR angiography; B) & C) in two different fluoroscopic views with a kinked 40 cm long 6 Fr sheath making advancement of the Symplicity II catheter impossible; D) & E) stepwise advancement of a 0.018 inch dummy wire straightening the kink of the sheath; F) successful passage of the denervation catheter through the sheath.
KINKING/BENDING OF THE ABDOMINAL AORTA
This vessel anomaly can result in the use of two differently shaped guiding catheter configurations as shown in Figure 10A, Figure 10D. For the right renal artery, a renal double curve configuration was appropriate whereas for engagement of the left renal artery an IMA configuration had to be used.
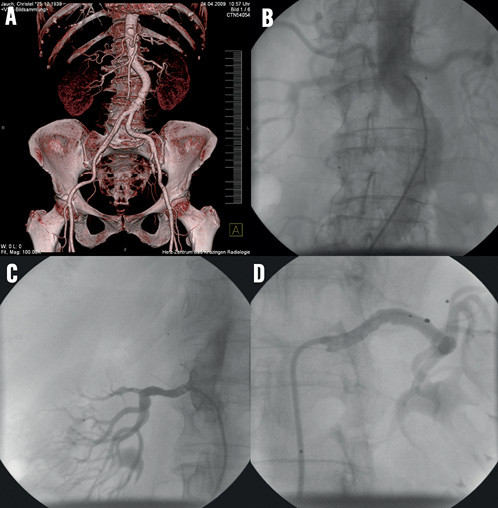
Figure 10. A) & B) CT angiography and conventional angiography of curved infrarenal abdominal aorta; C) & D) choice of two different dedicated guiding catheter configurations, RDC for the right renal artery, LIMA for the left renal artery.
HOSTILE AORTA
Diffuse soft plaque atheroma (Figure 11) or partially thrombosed aortic aneurysm imposes the risk of micro- or macro-embolism into the kidney with consecutive loss of kidney function during engagement of the renal artery origin with the guiding catheter. In such scenarios, alternative treatment techniques such as baroreflex activation therapy (BaroStim neoTM; CVRx, Inc., Minneapolis, MN, USA) should be considered for treatment of resistant hypertension. For highly skilled operators, the “proximal protection technique” could be an option to avoid complications (see above).
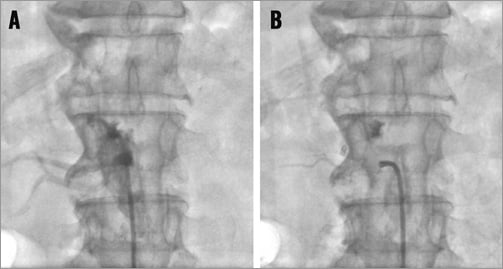
Figure 11. A) & B) “Hostile” aorta, contrast “staining” of the abdominal aorta after non-selective contrast injection through a guiding catheter as a sign for diffuse atheroma of the aortic wall with soft plaque being at high risk for cholesterol- and macro-embolisation.
ACUTE OFF-TAKE OF THE RENAL ARTERY
To overcome this access limitation three options exist: first, the dummy wire technique; second, the telescope technique (including the use of a dummy wire); and third, the brachial access, which is not approved by any company yet.
DUMMY WIRE TECHNIQUE If the renal artery off-take is too steep to gain entry with the denervation catheter a steerable extra support 0.014 or 0.018 inch guidewire with a flexible tip (e.g., Galeo ES; Biotronik AG, Berlin, Germany, or Cruiser 18; Biotronik AG) should be introduced into the distal renal artery. This usually results first in a reduction of the severity of the off-take angle and second in the option to advance the guiding catheter slightly into the renal artery origin (Figure 12; cave: atherosclerotic plaque!). Thereafter, the denervation catheter can be advanced into the distal renal artery. Important: before starting the radiofrequency energy application the guidewire tip must be pulled back at least a centimetre proximal to the location of the denervation catheter tip or completely removed; otherwise, the guidewire could conduct the energy.
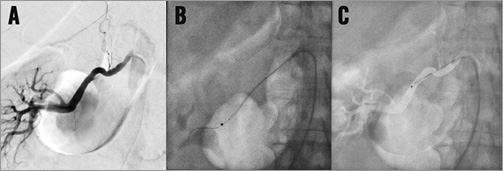
Figure 12. A), B) & C) Acute angled off-take of the right renal artery, dummy wire technique to facilitate the introduction of the denervation catheter.
TELESCOPE TECHNIQUE8 If the dummy wire technique does not enable the introduction of the denervation catheter into the renal artery the telescope technique might help. The first step is the intubation of the renal artery origin with a 5 Fr diagnostic catheter in Sidewinder or SOS configuration which is introduced through a 55 cm long guiding catheter in hockey stick configuration. A stiff 0.018 inch guidewire with a floppy tip is advanced into the renal artery. Thereafter, the guiding catheter is advanced over the 5 Fr diagnostic catheter into the proximal part of the renal artery trunk and the 5 Fr diagnostic catheter is removed (Figure 13).
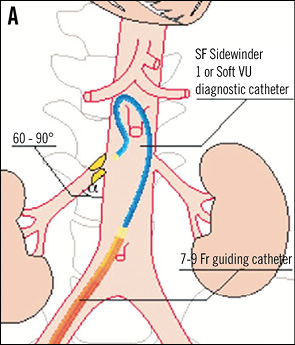
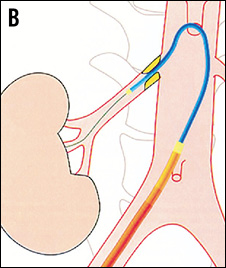
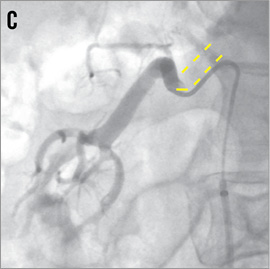
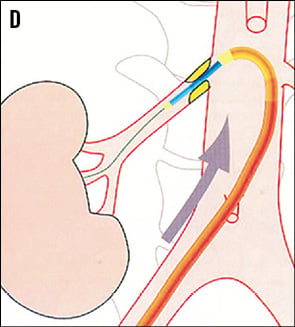
Figure 13. Renal artery cannulation in telescoping technique. A) Deep intubation of the renal artery origin with a 5 Fr diagnostic catheter (Sidewinder or SOS) through the 6 Fr guiding catheter. B) & C) A 0.014 inch or 0.018 inch extra-support guidewire is inserted into the renal artery; optionally, the lesion can additionally be crossed with the diagnostic catheter. D) The guiding catheter is advanced over the 5 Fr diagnostic catheter into the renal artery and the 5 Fr catheter is then removed. Cave: verify the absence of plaque at the origin of the renal artery.
BRACHIAL ACCESS If anatomical conditions do not permit the use of the femoral access a brachial access might be considered. Currently, no renal denervation device, in particular the Symplicity II catheter, is approved for the brachial access. The left brachial access is safer because no brain providing artery except the left vertebral artery has to be passed with a wire or catheter. Moreover, the distance to the renal arteries is shorter from the left side as compared to the right side. A 6 Fr sheath is introduced into the brachial artery via the antecubital fossa using a micropuncture technique. A dedicated 90 cm long Multipurpose guiding catheter, which is provided by some companies on demand (e.g., Cordis), is recommended. Of note: regular 100 cm long guiding catheters cannot be used due to the limited length of the catheter shaft of the Symplicity II catheter. Alternatively, a 90 cm long straight 6 Fr sheath (Cook, Bjaeverskov, Denmark) or a multipurpose shaped guiding sheath (VISTA BRITE TIP® IG, Cordis, J&J) can be positioned proximal to the origin of the renal artery. Thereafter, a 0.035 inch guidewire has to be navigated into the renal artery followed by a 6 Fr Multipurpose diagnostic catheter, which has to be positioned in the proximal segment of the renal artery. In the telescope technique the sheath is then advanced over the diagnostic catheter into the renal artery (Figure 14). With this stable sheath position maintained the denervation catheter can be easily advanced into the distal segment of the renal artery. Anchoring the sheath tip in the origin of the renal artery prevents the transmission of the aortic pulsation to the tip of the denervation catheter and guarantees a proper energy delivery to the vessel wall.
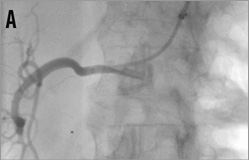
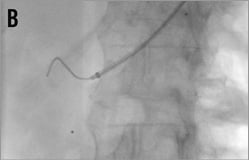
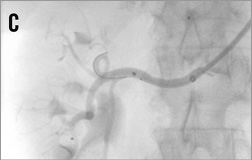
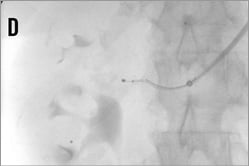
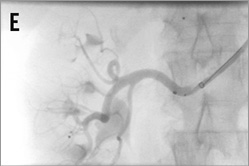
Figure 14. Brachial access, telescope technique: A) intubation of the right renal artery with a 5 or 6 Fr Multipurpose diagnostic catheter; B) advancement of the 6 Fr 90 cm long shuttle sheath (Cook) into the renal artery over the diagnostic catheter; C) 0.018 inch dummy wire in place, Symplicity II catheter advanced into the renal artery; D) & E) stepwise renal denervation procedure from distal to proximal.
TREATMENT OF RENAL ARTERIES WITH SHORT MAIN TRUNK
As long as the diameter of the renal artery main branches (segmental arteries of first order) is at least 4 mm both branches can be denervated safely; if one of the two main branches is smaller than 4 mm only one branch should be treated (Figure 15). If the main trunk is long enough, one or two final ablations should be performed in an inferior and superior orientation after having treated the branches. However, no data are available about the performance of renal denervation of renal artery branches: in particular, it is unknown if it might be sufficient treating only one of the main branches because such renal artery morphologies have been excluded from previous trials.
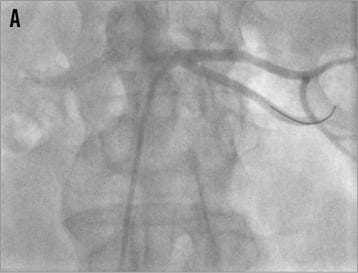
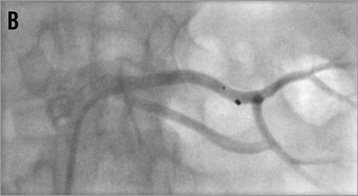
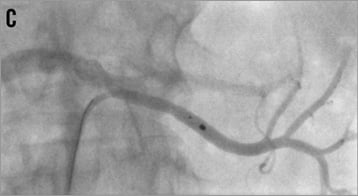
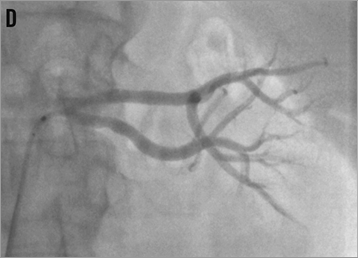
Figure 15. Percutaneous renal denervation procedure in a left renal artery with short main trunk. A) baseline angio; B) denervation of the superior branch; C) denervation of the inferior branch; D) final angiogram, mild spasm formation.
TREATMENT OF ACCESSORY ARTERIES
Similar rules can be applied to the treatment of multiple renal arteries as to the treatment of early bifurcating renal arteries. As long as the diameter of accessory arteries is at least 4 mm, renal denervation therapy can be performed safely. With decreasing vessel diameters the risk of severe spasm increases reciprocally (Figure 16). To date it is unknown if the treatment of accessory arteries increases the clinical treatment success.
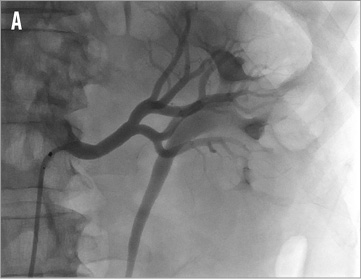
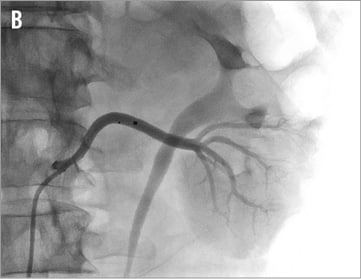
Figure 16. Accessory renal arteries: A) cranial main artery, ideal configuration for renal denervation therapy, vessel diameter 5 to 6 mm; B) caudal accessory artery, borderline diameter of 3 to 4 mm, renal denervation performed.
POTENTIAL COMPLICATIONS
Potential complications associated with renal denervation are summarised in Table 2.
Recommendations for avoiding complications are:
– No guiding catheter manipulation along the aortic wall without aspiration of potentially trapped debris.
– Use the appropriate access to the lesion. If indicated, change from a femoral to a brachial approach.
– Avoid unnecessary interventional steps. Every additional interventional step increases the risk of complication.
– Reduce the amount of contrast agent to that which is absolutely necessary. Consider diluting the contrast medium.
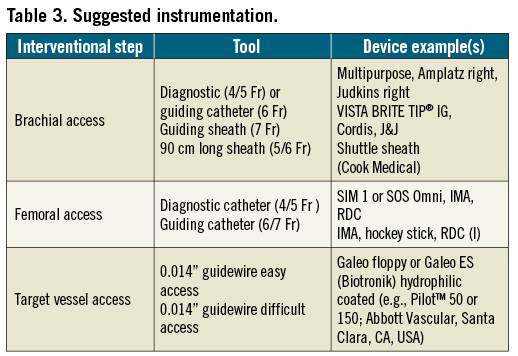
– Use the whole cardiovascular endovascular toolbox and techniques. If in doubt, ask a colleague of another specialty for support (Table 3).
Conclusion
Percutaneous renal denervation is an emerging interventional procedure with a rapidly increasing number of devices receiving CE mark. Currently available first-generation devices are limited by their stiffness, shaft length and some of them by being navigated without a guidewire. Thus, even if the standard case is usually a straightforward procedure, renal artery access can become a challenge under particular anatomical conditions. The knowledge of potential anatomical variations and appropriate interventional tools is an essential prerequisite for safe and successful interventional procedures.
Conflict of interest statement
T. Zeller is a member of the advisory board of Medtronic-Ardian. The other authors have no conflicts of interest to declare.

