Abstract
To most practitioners involved in catheter-based interventions, percutaneous renal sympathetic denervation is not technically challenging. However, under specific clinical circumstances (e.g., renal insufficiency) or when faced with more complex abdominal aortic anatomy (e.g., tortuosity) some procedural tips may come in handy. Here we review the equipment, antiplatelet and anticoagulant strategy as well as the procedural technique, including tips and tricks for the successful performance of catheter-based renal denervation. Among other topics, carbon dioxide angiography and brachial artery access are discussed.
Introduction
For most interventional cardiologists, nephrologists or interventional radiologists, the routine performance of percutaneous sympathetic renal denervation is not technically challenging. Some tips and tricks may, however, come in handy for a successful and safe procedure.
Angiography
AORTOGRAPHY
There are a number of techniques for abdominal aortic angiography. One option is to advance a pigtail or Omni Flush™ catheter (AngioDynamics. Inc., Queensbury, NY, USA) into the abdominal aorta over a 0.035 inch guidewire superimposing the tip on the transition between the first and second lumbar vertebrae followed by contrast injection (10-20 cc if digital subtraction angiography is used, 15-30 cc if conventional angiography is used). Alternatively, a 6 Fr guide catheter may be advanced over a 0.035 inch guidewire with the guide catheter tip between the first and second lumbar vertebrae with injection through the guide catheter while the wire remains in the distal thoracic aorta (this requires the use of a Y-connector prior to angiography). The same guide catheter may then be used to perform selective angiography. Some operators recommend a shallow (10-20 degrees) left anterior oblique projection for angiographic imaging given the slightly more anterior take-off of the right compared with the left renal artery. Close attention should be directed to size, take-off, course, tortuosity and bifurcation pattern of the renal arteries. Furthermore, the images should be scrutinised for the presence and size of accessory renal arteries and any significant atherosclerosis.
SELECTIVE GUIDE CATHETER ENGAGEMENT
Once the anatomy is characterised, an appropriate guide catheter is chosen. Most renal arteries have a slightly inferior take-off. Therefore, in the overwhelming majority of cases, the shape of an internal mammary catheter is most suitable for selective engagement. In cases of a horizontal take-off, a renal double curve guide catheter or a Judkins right may be well suitable. In the unusual case of a superior take-off, a multipurpose catheter may provide optimal alignment. In the absence of significant atherosclerotic abdominal aortic disease it is not unreasonable to proceed with direct guide catheter engagement. However, a safer technique, particularly in the presence of significant abdominal aortic atherosclerosis, may be the “no touch” technique. This entails maintenance of the 0.035 inch guidewire in the distal thoracic aorta while directing the guide catheter tip as close to the renal artery ostium as possible with injections of small amounts of contrast to confirm the close position of the guide catheter tip to the renal artery ostium. Once the catheter tip is near the ostium, the 0.035 inch guidewire is slowly withdrawn allowing the catheter to engage the renal artery more or less passively. This technique avoids undue guide catheter manipulation in the abdominal aorta, potentially reducing the risk of embolisation of atherosclerotic material. In addition, it may minimise the risk of guide catheter-induced renal artery dissection. Optimal guide catheter position is very important prior to insertion of most renal denervation devices. Positioning the tip 3-4 mm into the renal artery facilitates delivery of the renal denervation catheter as advancement to (and exit of the renal denervation catheter from) the guide catheter tip typically straightens the catheter slightly and may lead to disengagement. If engagement of the guide catheter proves challenging, consideration may be given to advance a glide wire carefully into the renal artery followed by a 4 Fr or 5 Fr diagnostic catheter as a rail for the guide catheter which, in turn, is gently advanced over the diagnostic catheter in a telescoping fashion.
SELECTIVE ANGIOGRAPHY
Two to three cc of contrast are typically sufficient for this purpose. It is important to allow extended cine-angiographic imaging for visualisation of the contrast nephrogram. Of note, selective renal angiography can be performed with less than a total of 10 cc of contrast under most circumstances with minimal risk of contrast-induced nephropathy (Figure 1). In case of advanced renal insufficiency, it is not unreasonable to forgo abdominal aortography accepting the chance of undetected small accessory renal arteries.
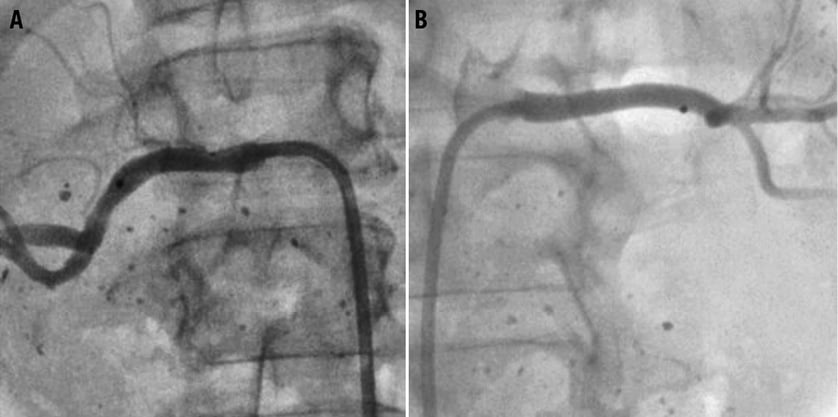
Figure 1. Due to advanced renal insufficiency, angiography and denervation were performed with the use of a total of 4 cc of contrast. Baseline selective left and right renal angiography were performed with 2 cc respectively (A and B). To conserve contrast, no aortogram or completion angiogram was performed. Hence, small accessory renal arteries may have been missed. Follow-up ultrasound did not show any significant renal artery stenosis.
CARBON DIOXIDE ANGIOGRAPHY
In the setting of advanced renal insufficiency or prior contrast allergy, performance of the entire procedure with carbon dioxide angiography can be considered (Figure 2). Carbon dioxide angiography typically produces an inferior imaging quality compared with conventional angiography. Nevertheless, it allows sufficient intraprocedural guidance for guide catheter engagement and renal denervation catheter positioning. Given the somewhat inferior performance for the detection of renal artery stenosis, alternative non-invasive imaging (e.g., with Duplex ultrasonography or non-contrast magnetic resonance angiography1-4) prior to the procedure can be considered.
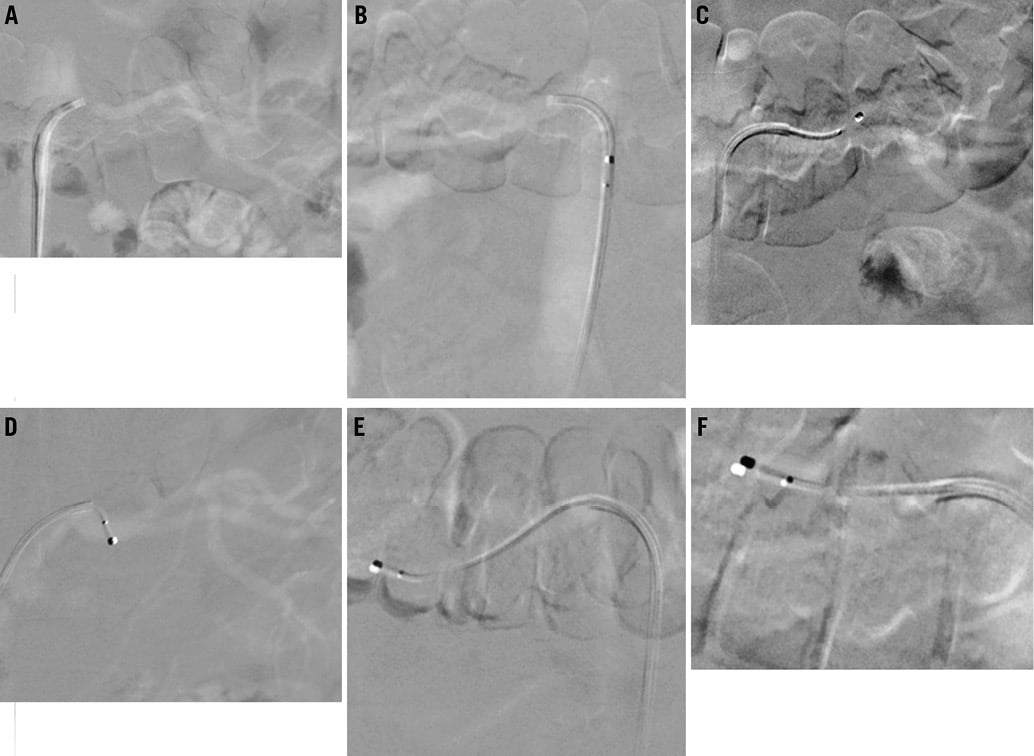
Figure 2. Selective carbon dioxide angiography of the left and right renal arteries respectively (A and B) as well as representative carbon dioxide angiography images with the Symplicity catheter positioned in the left (C and D) and right renal arteries (E and F).
Denervation
Prior to renal denervation catheter delivery, some operators routinely administer a vasodilator intra-arterially (e.g., 200-400 micrograms of intra-arterial nitroglycerine, 200 mcg of nicardipine, 100 mcg of verapamil or 100 mcg of nitroprusside) in an attempt to prevent or minimise vasospasm, however, others, including ourselves, do not routinely administer vasodilators because the prevention of vasospasm is questionable. For catheters using radiofrequency electrodes, a starting impedance of at least 220-250 ohm is desirable. Larger impedances indicate better catheter-wall contact. High impedance may indicate that the catheter position is in a small side branch. When the Symplicity™ catheter (Medtronic, Santa Rosa, CA, USA) is used, the following tips may help the performance of a successful procedure. If the starting impedance varies by more than 10-20 ohm, the catheter tip is not stable and its position should be modified. An impedance drop of >10% is expected and desirable. If this is not reached, a slight modification of the catheter tip position may be more likely to achieve the desired impedance drop. If an error signal leading to an interruption of radiofrequency energy application occurs during ablation, this is typically related to loss of catheter-wall contact requiring tip redirection to optimise contact. Alternatively, the catheter may be in a small side branch causing a significant temperature increase followed by interruption of the ablation. Ablations that are interrupted prematurely (prior to completion of two minutes) by error messages should be regarded as unsuccessful and not counted to the goal of four to eight ablations per artery. Meticulous care should be taken to flush the guide catheter (e.g., with heparinised saline) periodically to avoid thrombus formation within the catheter.
Spasm and luminal irregularities after ablations are common (Figure 3). Given histological reports of the renal arteries from animal studies with optical coherence tomography after renal denervation5 and autopsy specimen after radiofrequency application to the pulmonary veins6, the luminal changes likely correspond to vessel wall oedema, endothelial denudation and adherent thrombotic material. Occasionally, spasm may improve with the use of vasodilators, but, more likely than not, it will not improve the angiographic appearance. Under these circumstances it is important to resist the temptation to perform balloon angioplasty or stenting as these changes will (with the rare exception of development of renal artery stenosis7,8) invariably resolve (Figure 3).
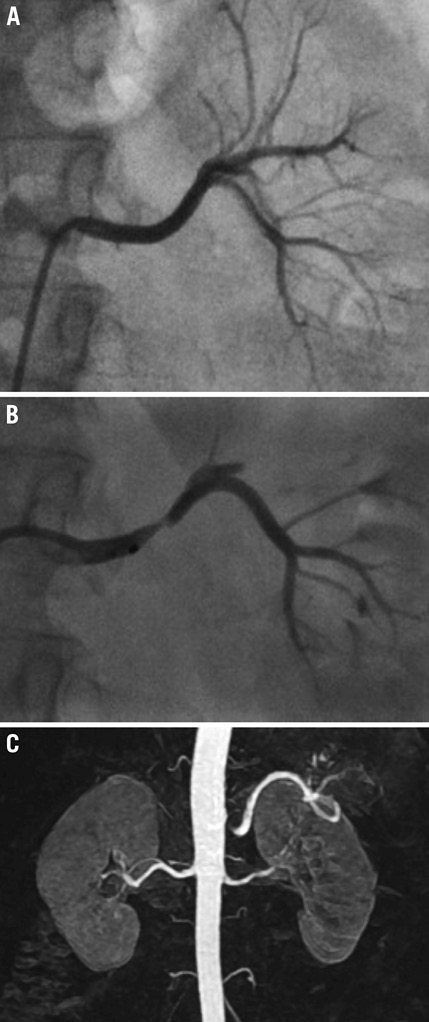
Figure 3. Baseline left renal arteriogram demonstrating an angiographically normal left renal artery (A). After several ablations, there is significant spasm and oedema of the arterial wall (B). Vasodilator was not accompanied by any significant improvement. Follow-up magnetic resonance angiography shows no significant renal artery stenosis (C).
Traditionally, (in the Symplicity trials) patients with accessory renal arteries were excluded from participation9,10. In addition, only arteries with a diameter of ≥4 mm were subject to renal denervation. In our experience, renal arteries with diameters of ≥3.5 mm are amenable to ablation with the Symplicity catheter; however, it should be mentioned that ablation in arteries with diameters <4 mm is currently considered an off-label manoeuvre using this catheter. In addition, in patients with accessory renal arteries, a blood pressure reduction can be achieved in those whose accessory renal arteries are also denervated, albeit perhaps less pronounced than reported in patients with renal denervation of bilateral single renal arteries11. Some novel devices may be used in smaller arteries and this will be outlined in other contributions to this Journal describing individual devices.
Some renal arteries have an early bifurcation. If both branches are amenable (of adequate size) they should both be treated.
Adverse anatomy
Iliac and/or abdominal aortic tortuosity may cause enough friction in the guide catheter that advancement of the renal denervation catheter into the renal artery may not be possible. In this case a long (e.g., 45 cm) sheath may be used to straighten the iliac/aortic vasculature and mitigate friction. Alternatively, brachial access may be considered (Figure 4). If the renal artery has an acute angle inferior take-off, guide catheter engagement and support may be suboptimal causing the guide to back out upon renal denervation catheter advancement. In this case, insertion of a 0.014 or 0.018 inch buddy wire into the distal or segmental renal artery may be helpful to offer more support and a stable guide catheter position. It is important to remember to remove the buddy wire during ablation. A sidewinder or Simmons catheter may be helpful in engaging an acute angle inferior renal artery take-off via the femoral approach followed by guide catheter advancement using the engaged diagnostic catheter as a rail.
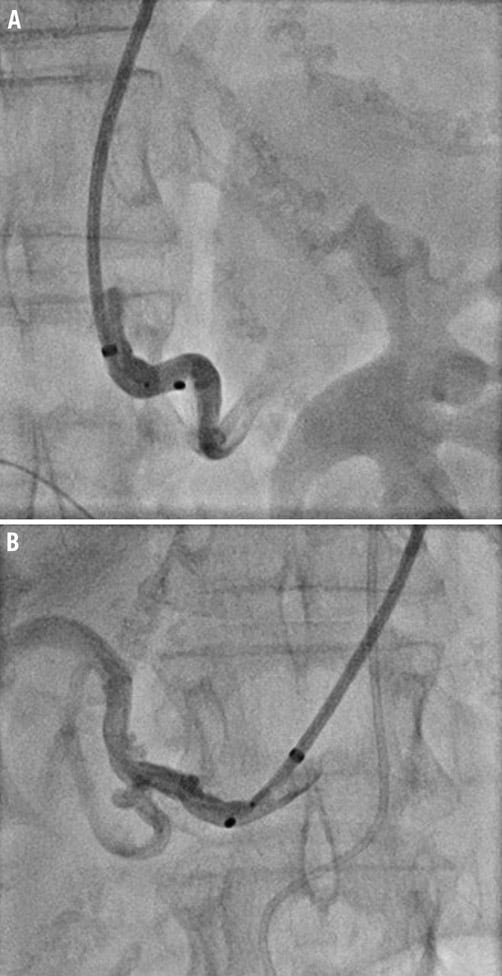
Figure 4. The left (A) and right (B) renal arteries are engaged with a 6 Fr 90 cm sheath via the right brachial artery due to excessive iliac tortuosity making renal denervation via the femoral approach impossible.
Renal denervation via the brachial approach
In some cases (excessive iliac and/or abdominal aortic tortuosity, extreme acute angle inferior take-off of the renal artery or abdominal or iliac occlusive disease), the only access options are the upper extremities. It is important to recognise that the Symplicity catheter is 100 cm long. Therefore, radial access typically will not allow this catheter to reach the renal arteries and brachial access is therefore preferred. In addition, most conventional guide catheters are 100 cm or longer and, hence, cannot be used (the Symplicity catheter will not exit the guide). The only feasible approach is the use of a 90 cm sheath or guide catheter. In this case, to assure atraumatic sheath engagement, first a diagnostic catheter (e.g., multipurpose) is advanced through the sheath and the renal artery is engaged with the diagnostic catheter followed by advancement of the sheath using the diagnostic catheter as a rail. When approaching the renal arteries from the upper extremities it is important to understand that respiratory motion and movement of the heart and thoracic aorta can lead to an unstable catheter position complicating the ablation by not allowing consistent catheter-wall contact. This motion is more pronounced with right than with left brachial access. For this reason, in addition to the frequently encountered tortuous right subclavian anatomy, the left brachial artery is preferred over the right.
Renal denervation in the presence of a renal artery stenosis or renal artery stent
When a renal artery stenosis is encountered in a patient with resistant hypertension the renal artery stenosis may be treated with balloon angioplasty and renal denervation performed at a later time if the blood pressure does not reach goal. It is important to avoid ablation in a stented segment as this may cause significant heat-related tissue injury. It is worth mentioning that, though a case of renal denervation in the presence of a renal stent has been reported12, prior renal stenting or the presence of a significant renal artery stenosis (>50%) had been a contraindication in both Symplicity trials and is currently not recommended13.
Other equipment
Apart from the previously mentioned guide catheters and sheaths as well as 0.035 inch J-tipped guidewires, equipment that allows renal artery stenting in the rare scenario of guide or renal denervation catheter-induced renal artery dissection should be readily available. This should include stents of 4-7 mm diameter (the typical diameter of renal arteries) and 10-20 mm length as well as 0.014 or 0.018 inch guidewires. The use of digital subtraction angiography is not imperative but may allow a smaller contrast amount. Pulse oxymetry for continuous oxygen monitoring as well as reversal agents (1 mg of intravenous flumazenil and 2 mg of intravenous naloxone) and emergent airway management equipment (including for intubation) in case of excessive sedation with respiratory compromise must be in the catheterisation laboratory. Personnel trained in emergent airway management must be readily available.
Sedation, analgesia and other necessary medications
Most renal denervation procedures are accompanied by visceral (abdominal, lower back or pelvic) pain. Hence, routine administration of benzodiazepines and opioid analgesics is necessary. Typically, intravenous midazolam and fentanyl are used (incremental administration of up to or 4 mg and 150 mcg, respectively, are not unusual for pain control). Some operators prefer the use of piritramide (7.5 mg intravenously at the initiation of the procedure followed by 3 mg boluses) or morphine over other opioids. Further, some advocate pregabalin 50 mg orally the day before and the day of the procedure and/or 1 gr of intravenous novalgin one hour prior to the procedure. Antiemetics (e.g., 1 mg intravenous granisetron) may be used to prevent opioid-associated nausea. Intravenous atropin (1 mg) should be available in case of a vagal reaction as well as flumazenil and naloxone to reverse benzodiazepine and opioid actions in case of respiratory depression.
Conflict of interest statement
H. Sievert’s institution has ownership interest in or has received consulting fees, travel expenses or study honoraria from the following companies: Abbott, Access Closure, AGA, Angiomed, Arstasis, Atritech, Atrium, Avinger, Bard, Boston Scientific, BridgePoint, Cardiac Dimensions, CardioKinetix, CardioMEMS, Coherex, Contego, CSI, EndoCross, EndoTex, Epitek, Evalve, ev3, FlowCardia, Gore, Guidant, Guided Delivery Systems, Inc., InSeal Medical, Lumen Biomedical, HLT, Kensey Nash, Kyoto Medical, Lifetech, Lutonix, Medinol, Medtronic, NDC, NMT, OAS, Occlutech, Osprey, Ovalis, Pathway, PendraCare, Percardia, pfm Medical, Rox Medical, Sadra, Sorin, Spectranetics, SquareOne, Trireme, Trivascular, Velocimed, Veryan. The other authors have no conflicts of interest to declare.

