Abstract
Aims: Renal artery denervation (RAD) is an effective treatment for resistant hypertension. The Navigation and Visualisation Technology (NavX) system creates three-dimensional (3-D) anatomical maps to guide catheter position and identify ablation sites. This first-in-human study assessed the utility of NavX 3-D mapping for RAD.
Methods and results: Consecutive patients who underwent RAD using fluoroscopy alone (control group, n=8) were compared to NavX-guided RAD (NavX group, n=10). Where NavX was utilised, orthogonally located skin patches were applied to the torso for acquisition of 3-D geometry of each renal artery. Baseline clinical characteristics and renal artery anatomy were similar between groups. Median contrast dose was significantly higher in the control versus NavX group (123 ml versus 78 ml, p=0.016). Median radiation dose was significantly higher in the control versus NavX group (166 Gy cm2 versus 43 Gy cm2, p=0.034). Mean changes in systolic and diastolic BP at three-month follow-up were –22/–9 mmHg and –23/–11 mmHg in the control and NavX group, respectively (p=0.99).
Conclusions: Use of NavX mapping as compared to standard renal artery denervation was found to correlate with a significant reduction in contrast load and radiation exposure. This study demonstrates both the feasibility and potential benefits of NavX 3-D mapping to guide renal artery denervation.
Introduction
Renal artery denervation (RAD) has been shown to be an effective treatment for patients with resistant hypertension (HTN)1, with the long-term efficacy and safety confirmed in the randomised Symplicity HTN-2 trial2. However, 10% of patients undergoing RAD still do not respond, and a larger proportion have only a partial BP response2. Traditional two-dimensional fluoroscopy to guide RAD requires contrast injections to identify the renal artery position within the abdominal aorta, and also to demonstrate catheter position prior to each ablation. As a result, patients with an eGFR ≤45 ml/min have been excluded from all the major trials due to the potential risk of contrast-induced nephropathy. The NavX™ (Navigation and Visualization Technology; St. Jude Medical, St. Paul, MN, USA) system is a three-dimensional (3-D) mapping tool commonly used in cardiac electrophysiologic ablation procedures. Typically utilised for arrhythmia mapping and ablation, it involves placement of electrode patches on the patient’s skin. These patches transmit a low-level current which is recorded by an electrode on the ablation catheter within the cardiac chambers. The recorded voltage and impedance allow the distance from each skin patch (and as a consequence, catheter location in space) to be calculated. The NavX system collects data points from the catheter and creates detailed 3-D geometry of the heart, in order to track catheter movement and mark ablation sites. In cardiac ablation procedures, 3-D mapping systems have been found to reduce fluoroscopy time and radiation dose significantly3,4. The use of NavX in renal artery denervation is novel and has not been described previously. We hypothesised that NavX mapping could be used to guide catheter ablations within the renal artery. Once the geometry of the renal artery was obtained, no further fluoroscopy or contrast injections would be needed to determine catheter position or identify ablation sites. NavX 3-D visualisation could therefore allow circumferential and longitudinal placement of ablation sites with the potential to reduce contrast load and radiation exposure.
Methods
This study was approved by the Westmead Hospital Ethics Committee and all participants gave their informed consent. Patients were eligible if they had confirmed resistant HTN (systolic blood pressure of 150 mmHg or more) despite being treated with at least three antihypertensive medications, were not pregnant, were aged >18 years and did not have any known secondary cause of hypertension. Patients with renal artery stenosis (all patients required some type of renal artery imaging to be excluded) and previous renal stenting/angioplasty were excluded. Patients were referred and booked for RAD after being assessed by an independent investigator to ensure eligibility criteria were met. Consecutive patients then underwent fluoroscopy-guided RAD from September 2011 to January 2012, followed by NavX-guided RAD from February to September 2012. Prior to the commencement of this study the proceduralist was already experienced with RAD. Patients were followed as in-patients and then at three and six months following the ablation procedure with assessment for adverse events, medication use and measurements of home and office-based blood pressure. The primary outcomes were feasibility of NavX, and effect of NavX use on procedural contrast and radiation doses. Secondary outcomes were blood-pressure lowering effectiveness of RAD utilising NavX, and safety, which included procedural complications and major adverse cardiovascular events defined as death, myocardial infarction, heart failure and stroke.
Procedural details
RAD was performed in the cardiac catheterisation laboratory by a single operator who was an interventional cardiologist. A vascular surgeon was also present who had extensive endovascular experience. A 6 Fr sheath was placed in the femoral artery to allow access to the aorta and renal arteries. An aortogram was performed to locate the origins of the renal arteries. Once selectively engaged, renal arteriography was performed pre-ablation to confirm the absence of significant vascular abnormality, including obstructive renal artery disease (≥50% stenosis) or angiodysplasia. Patients with dual renal arteries were not excluded. A radiofrequency catheter (Symplicity catheter; Medtronic Inc., Minneapolis, MN, USA) was introduced into each renal artery and four to eight radiofrequency ablations were performed in each artery lasting up to two minutes each, and of eight watts or less. During ablation the catheter system monitored for an increase in tip temperature and impedance to ensure adequate contact. Angiographic evaluation after renal denervation was performed to confirm no compromise of treated arteries. Measurements of contrast and radiation dosage were recorded by an independent observer according to a predefined measurement protocol. Intravenous infusion of fentanyl and midazolam were used to sedate the patients and provide analgesia for visceral abdominal pain associated with denervation.
NavX mapping
The procedural and technical set-up for NavX-guided RAD is shown in Figure 1. Where 3-D mapping was utilised, a total of six orthogonal skin electrode patches were placed on the patient. In order to concentrate the Ensite Enguide™ (St. Jude Medical) impedance field within the boundaries of the renal anatomy and to minimise respiratory and abdominal motion artefact, the skin electrodes were placed according to a modification of the guidelines recommended for cardiac mapping (Figure 1B). Originally, in a pilot study, the lateral and anterior patches were placed on the abdomen at the level of the renal arteries. However, this non-standard approach was abandoned as diaphragmatic motion significantly impacted on the mapping accuracy. In all study cases, respiratory motion artefact was automatically corrected for by the NavX system. The combination of adequate intravenous sedation, automated respiratory motion compensation and electrode patches adherent to the patient rather than an external fixed reference reduced variation anatomy geometry due to patient motion. Once the renal artery was selectively engaged, 3-D geometry was acquired on the NavX system by navigating the catheter from the bifurcation to the ostium. High-density mapping points were continually and rapidly collected in real time as the catheter was moved, without any intervention from the proceduralist. Specific sites (in 3-D) were tagged for radiofrequency ablation and annotated on the geometry collected using NavX. Where available, magnetic resonance angiography (MRA) was merged with NavX to create the 3-D images.
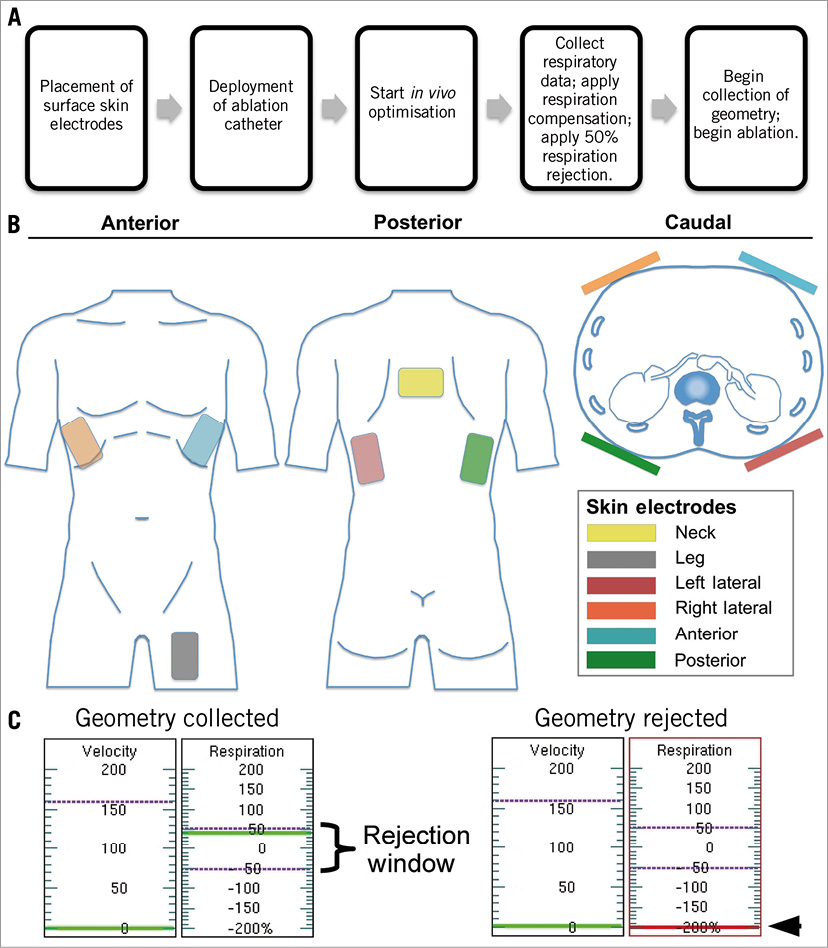
Figure 1. A) Procedural set-up for NavX-guided renal artery denervation with B) placement of skin electrodes, and C) automatic respiratory motion correction.
Calculation of renal artery tortuosity index
For each patient, the tortuosity index of each renal artery, based on fluoroscopic cine frames, was determined using the arc:chord ratio method. The widely used arc:chord formula5 was adapted for our study as follows:
![]() ,
,
where t=tortuosity index, la=length of the anterior arc length of the renal artery spanning the ostium to the distal bifurcation, li=length of the inferior arc length of the renal artery spanning the ostium to the distal bifurcation, and c=straight-line distance between the ostium and distal bifurcation. For each set of measurements, distances were calibrated based on the known fixed distance of the catheter distal electrode and proximal marker, each of which was radiopaque. Calculation of the tortuosity index is illustrated in Figure 2.
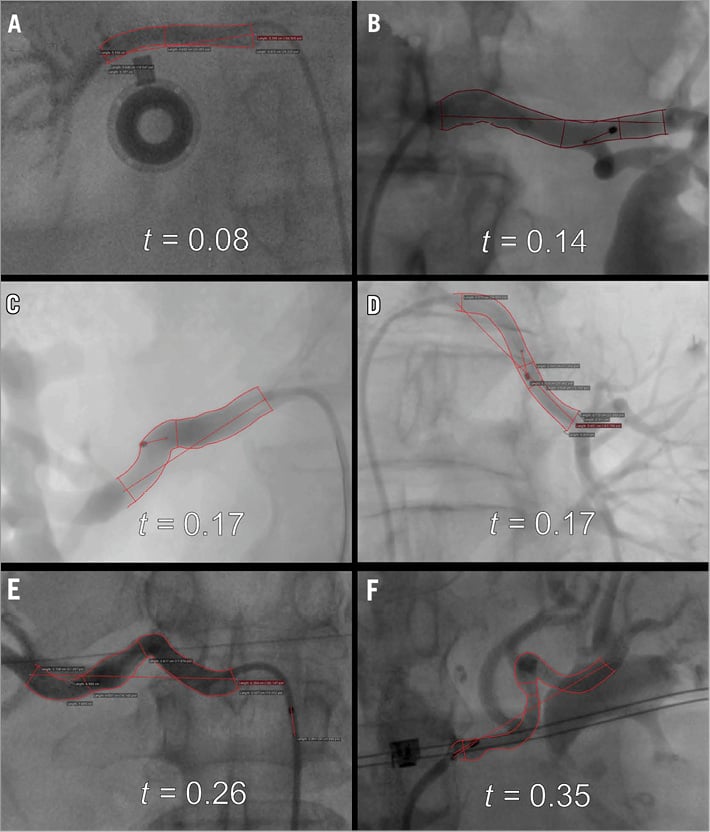
Figure 2. Example of anatomical variations observed using fluoroscopic imaging, and relative degree of tortuosity measured. A) & B) Example of non-tortuous renal anatomy. C) & D) Example of moderately tortuous renal anatomy. E) & F) Example of highly tortuous renal anatomy. Left panels: right renal arteries. Right panels: left renal arteries.
Statistical analysis
SPSS for Windows (release 21.0; SPSS Inc., Chicago, IL, USA) was used to analyse the results. Two-tailed tests with a significance level of 5% were used throughout. Chi-square or Fisher’s exact tests as appropriate were used to test for association between categorical variables. Mann-Whitney tests were used to analyse for differences between continuous variables.
Results
A total of eight patients underwent RAD using fluoroscopy alone (control group, n=8) while 10 patients underwent RAD with the use of NavX (NavX group, n=10). NavX was successfully utilised in all patients in whom it was attempted. An example of the NavX mapping, anatomical geometry and distribution of ablation sites as compared to fluoroscopy is shown in Figure 3. A single patient in the NavX group also had an MRA pre-RAD which was incorporated into the NavX system (Figure 4).
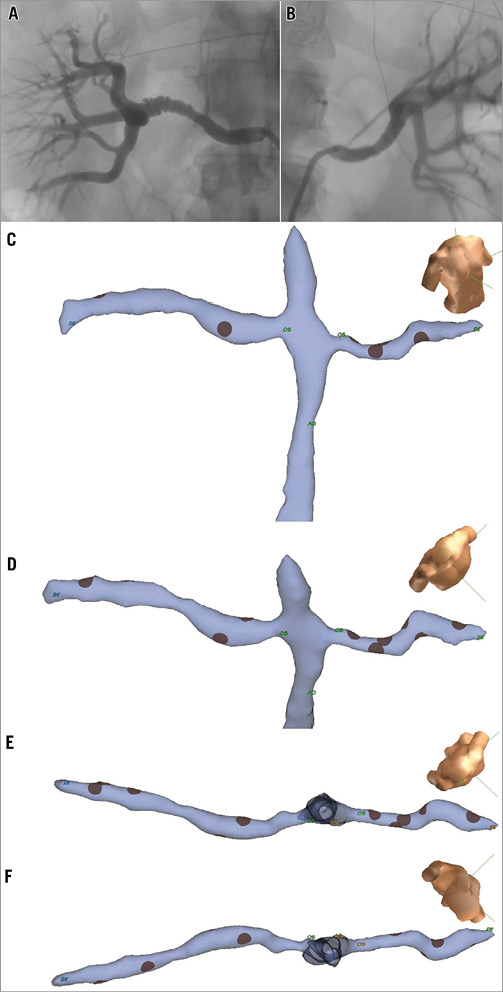
Figure 3. Fluoroscopy image of A) right and B) left renal artery angiography in anteroposterior projection. NavX geometry created for the same patient demonstrating right and left renal arteries and abdominal aorta in C) anterior projection, D) anterocranial projection, and renal artery geometry from E) cranial, and F) caudal projection.
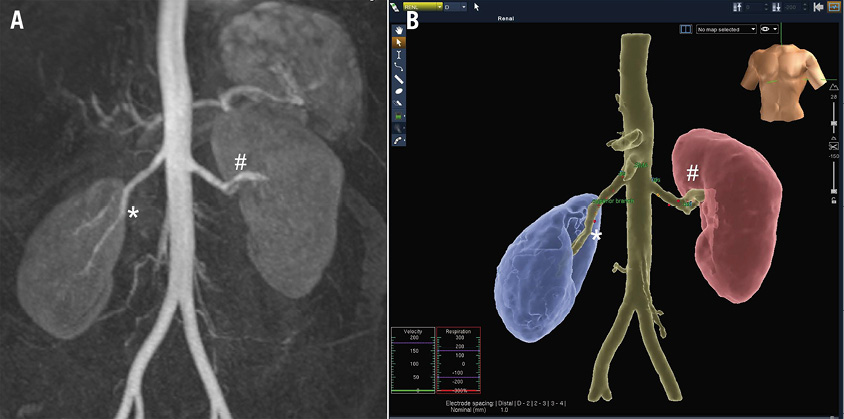
Figure 4. A) Gadolinium-enhanced magnetic resonance angiography (MRA) of abdominal aorta and renal arteries. B) MRA/NavX fusion image demonstrating ablation sites within both renal arteries mapped and annotated.
Baseline characteristics
The overall cohort (n=18) had a median age of 64 years and was taking a median of five antihypertensive medications. Mean baseline blood pressure (BP)±SD was 187±16/94±19 mmHg in the control group and 173±21/85±10 mmHg in the NavX group (p=0.14). There was no significant difference in the baseline clinical characteristics of the two groups including age, gender, BMI, renal function, number of antihypertensive medications or background medical problems (Table 1).
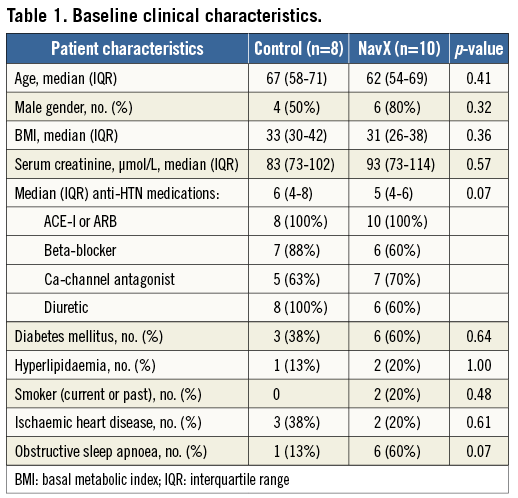
Procedural details
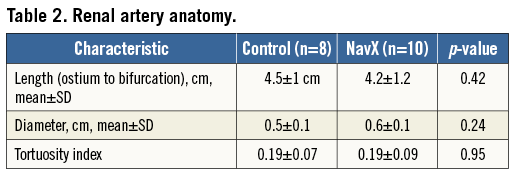
The renal artery anatomy was similar between the two groups in terms of length, diameter and tortuosity (Table 2). The procedural characteristics are shown in Table 3. A median of six ablations per artery was performed in both groups. There was a higher amount of midazolam used to sedate patients in the NavX group. In two patients where the renal artery position was not adequately identified after an aortogram and was difficult to engage selectively, NavX was used to map the aorta and guide catheter movement to the renal artery ostium.
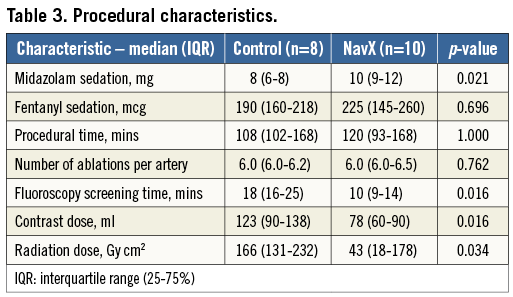
Contrast and radiation doses
The contrast and radiation doses are shown in Table 3. The median contrast dose, fluoroscopy screening time and radiation dose were all significantly higher in the control versus NavX group.
Safety outcomes
There were no major procedural complications in either group. A single patient in the NavX group had a small femoral haematoma post-procedure which was treated conservatively. On follow-up at three months, all patients were alive with no occurrence of any major adverse cardiovascular events.
Blood pressure outcomes
Mean changes in systolic and diastolic BP at discharge were –40/–15 and –24/–3 mmHg in the control group and NavX group, respectively (p=0.22). Mean changes in systolic and diastolic BP at three-month follow-up were –22/–9 mmHg and –23/–11 mmHg in the control group and NavX group, respectively (p=0.99). In those patients who completed six-month follow-up (n=14), mean changes in systolic and diastolic BP at six months were –22/–9 mmHg and –30/–11 mmHg in the control group and NavX group, respectively (p=0.69).
Antihypertensive medications
At baseline, patients in the control group were taking a mean of 6.1±1.8 antihypertensive medications which did not change at follow-up with a mean of 6.2±2.2 and 6.0±2.2 antihypertensive medications at three and six months, respectively. At baseline, patients in the NavX group were taking a mean of 4.5±1.8 antihypertensive medications which did not change at follow-up with a mean of 4.3±1.8 and 5±1.0 antihypertensive medications at three and six months, respectively. A total of nine out of 18 patients changed their antihypertensive medications during follow-up. This included three patients in whom antihypertensive medications were increased (one patient in the control group, two patients in the NavX group) and six patients in whom antihypertensive medications were reduced (three patients in the control group and three patients in the NavX group).
Discussion
This first-in-human study demonstrated the feasibility of NavX to create 3-D geometry of the renal arteries and guide RAD. The use of NavX in RAD resulted in a significant reduction in both contrast load and radiation dose.
In this study, NavX was used to create 3-D geometry of the renal arteries, which was then used to guide the Symplicity catheter within the artery. Once the ostium of the renal artery and the site of bifurcation were marked on NavX, ablation sites were easily positioned between these two points. During the procedure, each ablation site was marked on the NavX system, allowing optimal distribution of ablations along the artery as the catheter was retracted.
NavX mapping was found to reduce the contrast dose used in RAD significantly. This reduction was largely a result of renal artery 3-D geometry display which enabled contrast to be spared, which would otherwise have been injected to demonstrate the catheter’s position prior to ablation. It is difficult to know how contrast dose in the current study compares to that utilised in other studies. A small study of renal denervation in 15 patients with stage 3-4 chronic kidney disease demonstrated a reduction in contrast dose from 83 ml to 47 ml through the use of CO2 angiography6. However, the amount of procedural contrast used in the major Symplicity HTN-1 and HTN-2 trials was not reported1,2. In our study, we found that a large amount of contrast was typically used at the start of the procedure in order to identify renal artery position in the abdominal aorta. This was done with aortography followed by repeated local contrast injections. In two patients where renal artery ostia were not well visualised on fluoroscopy, NavX was used to map the aorta and assist with selective catheter engagement. In these two patients NavX mapping enabled catheter guidance to engage the renal artery selectively without repeated local contrast injections under fluoroscopy. Abdominal aortic mapping, if performed in all patients, could potentially reduce contrast load even further, but would require more catheter manipulation within the aorta.
Similar to cardiac ablation procedures, we found that anatomic mapping to guide renal ablations significantly reduced fluoroscopic exposure and radiation dose3. In cardiac ablation procedures, CT or MRI images of the cardiac structures are frequently merged with 3-D mapping systems such as NavX and CARTO in order to obtain more detailed anatomy7. Ideally, patients undergoing RAD could undergo magnetic resonance angiography (MRA) with gadolinium to identify renal artery origin and anatomy (Figure 1). The MRA can be merged with the mapping system during the ablation procedure. We found that the benefits of merging MRA images with the mapping systems included easier identification of the location of the renal artery ostium, demarcation of the bifurcation, assistance in selecting the larger renal artery in the presence of dual renal artery anatomy and aid in separating ablation lesions in a 3-D manner. MRA-NavX merging can potentially limit the fluoroscopy and contrast dose even further.
We found that there was no significant increase in our median procedural time in the NavX group versus the control group. However, in most institutions RAD is performed by interventional cardiologists who typically have little or no experience with mapping techniques and this could add increased set-up time. In the current study the NavX system was separate from the RAD system. In the future, RAD and NavX could be incorporated into a single system which would simplify the procedure and shorten any additional preparation time, with no additional training required for the proceduralist. Incorporation of NavX into RAD does result in an increased cost through the use of electrode patches as well as the additional personnel needed. This additional expenditure would need to be weighed against the possible benefits of reduction in length of stay associated with acute renal failure or the need for dialysis once RAD use is expanded to the cohort of patients with renal impairment.
There was no difference in blood pressure or antihypertensive use at three and six-month follow-up in the NavX versus control patients. This study was not powered to detect such a difference, but instead was designed to assess the feasibility of NavX and its impact on the short-term procedural characteristics. Overall, RAD was found to result in a reduction in mean systolic and diastolic BP post procedure similar to that seen in the Symplicity HTN trials1,2, which persisted at six-month follow-up.
Renal catheter denervation is a new and rapidly emerging area, with the current technology still in its infancy. Multielectrode catheters are in the process of being developed to ablate multiple sites within the renal artery simultaneously. These multielectrode catheters monitor temperature and impedance at each site, with the ablation at a site discontinued if there is poor contact with the renal artery wall. In fluoroscopy-guided RAD it would not be clear which site was not effectively treated. However, if NavX were utilised, the sites not ablated would be marked, and further manual ablations in this area could be performed. In addition, multiple electrodes on the new multielectrode catheter could be used simultaneously to map renal artery and aortic geometry in the NavX system, enabling faster mapping, with more accurate volumetric representation of the arteries. Utilisation of NavX with new multielectrode catheters could potentially speed up the procedure as well as increase its safety. Given the rapidly increasing number of patients undergoing RAD, it is also possible that patients will undergo repeat ablation procedures. NavX mapping in such patients would be invaluable to demonstrate previous ablation sites and enable re-do ablations.
Conclusion
Use of NavX mapping for renal artery denervation is not only feasible but, when utilised, can significantly reduce contrast load and radiation exposure. This 3-D image display technology has the potential to expand RAD use to the large population of patients with resistant hypertension and concurrent renal failure.
Limitations
The main limitation of this study was that it was not randomised and the operator was not blinded, which may have introduced bias. However, despite the study not being randomised, the two patient groups were well matched. The second limitation was the small sample size. However, we were still able to demonstrate the benefits of NavX in reducing contrast and radiation. In addition, we were able to fulfil our primary aim, which was to demonstrate that NavX is feasible to guide RAD. Lastly, the study has not demonstrated a superior efficacy of NavX, compared to fluoroscopy alone, in reducing long-term BP with RAD. However, this was not the aim of this feasibility study, and would require randomisation of thousands of patients.
Conflict of interest statement
The authors have no conflicts of interest to declare.

