Abstract
Aims: In small clinical trials, sympathetic renal denervation using radiofrequency (RF) energy shows promise in treating resistant hypertension. However, the RF procedure is lengthy and is associated with pain during ablation. Vascular brachytherapy, a proven treatment for in-stent restenosis, has the potential to cause nerve fibrosis. The purpose of the present study was to assess the safety and feasibility of renal artery brachytherapy for sympathetic renal denervation.
Methods and results: A total of 10 normotensive domestic swine underwent vascular brachytherapy to left and right renal arteries using the Beta-CathTM 3.5 Fr delivery system at doses of 25 Gy (n=8) and 50 Gy (n=8) at 2 mm from the source centre. These groups were compared to untreated arteries that served as control (n=4). Follow-up obtained at one or two months included angiogram, intravascular ultrasound, and histopathology analysis. The vascular brachytherapy procedure was safe and no apparent angiographic or ultrasound injuries to the vessel were seen. Histology showed a varying degree of thermal injury more pronounced in the 50 Gy group. The majority of examined nerves showed some degree of injury; there was a dose-related effect on nerve injury severity. There were varying degrees of arteriolar changes in the examined sections, with most showing a 2-20% degree of endothelial cell loss.
Conclusions: This initial feasibility and safety study of renal nerve denervation, mediated by low and intermediate β-radiation dosages, indicates that this approach can cause nerve fibrosis while avoiding significant damage to the renal artery.
Introduction
Hypertension remains a public health concern despite major advances in drug therapies over the last two decades. In the United States, nearly one third of the population has hypertension and the global prevalence continues to climb, particularly in developing countries1,2. A large body of evidence supports the tight relationship between the sympathetic nervous system and hypertension. Accordingly, surgical thoracolumbar sympathectomy was historically used to treat patients with severe hypertension3,4; however, this procedure was associated with significant morbidity. More recently, sympathetic renal denervation was obtained by using percutaneous radiofrequency (RF) ablation in the renal artery. This method is currently undergoing evaluation in clinical trials5. Today, when used clinically, RF ablation is associated with an overall treatment time of nearly 45 minutes and is associated with pain to the patient, thereby requiring heavy sedation. Vascular brachytherapy (VBT) is currently approved for the treatment of in-stent restenosis and has the potential to cause nerve fibrosis, which was seen in an experimental animal model in which brachytherapy induced linear lesions of fibrosis to the conduction system for the treatment of atrial fibrillation. The aim of the present study was to assess the feasibility and safety of a novel intravascular beta radiation approach for sympathetic renal denervation. In addition, as proof of concept, we attempted to demonstrate a dose response and to detect an optimal radiation dose for this indication.
Methods
ANIMALS AND STUDY DESIGN
Animal procedures were approved by the institutional Animal Care and Use Committee and performed according to contemporary National Institutes of Health guidelines. Ten Yorkshire swine were used.
ANIMAL PROTOCOL
Animals (41±3 kg) were anaesthetised, intubated, and provided mechanical ventilation throughout the procedure. Vascular access was obtained using a sterile carotid artery cut-down technique. VBT was performed with the Beta-CathTM 3.5 Fr delivery system (Novoste, Best Vascular, Inc., Springfield, VA, USA) (source train length of 40 mm, 16 source seeds), which was delivered into the target renal artery through a guiding catheter over a 0.0014” balanced middle-weight guidewire positioned along the main renal artery. At the end of the brachytherapy treatment a final angiogram was performed.
DOSIMETRY DETAILS
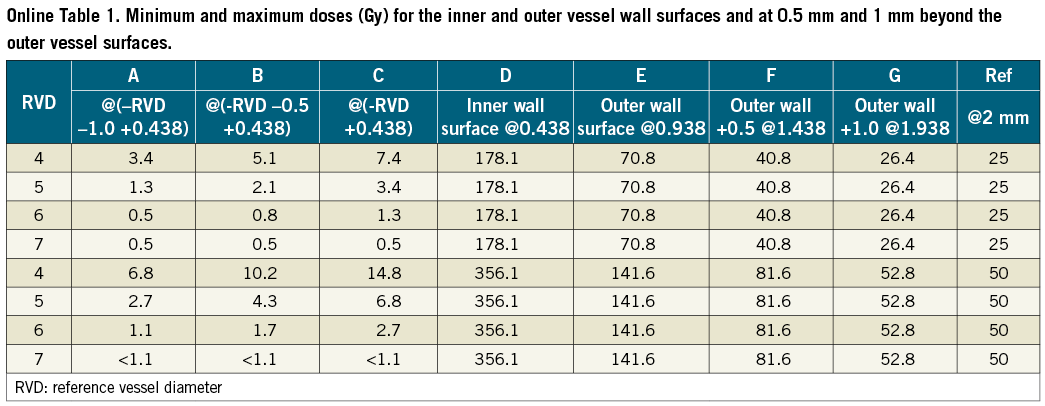
The Beta-Cath delivery catheter (Novoste) was utilised. The system contained a 40 mm long source train of strontium/yttrium [Sr/Y]-90 delivered hydraulically via a closed end lumen over the wire catheter placed at the left or right renal artery. A dose of 25 or 50 Gy at 2 mm from the source centre was prescribed. During use, the source train is coaxial with the inner and outer delivery catheter lumens. For minimum and maximum dose modelling, the position of the source train relative to the inner and outer member catheter lumens, in axial views for minimum and average distances, is shown in Figure 1. The minimum and maximum doses for the inner and outer vessel wall surfaces, and at 0.5 mm and 1 mm beyond the outer vessel wall surfaces, are shown in Online Table 1 and Online Figure 1.
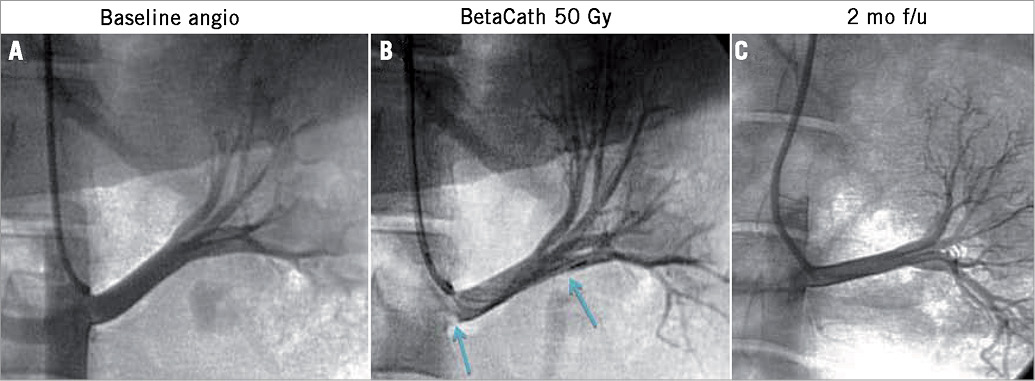
Figure 1. Renal artery angiography at baseline and up to two months post brachytherapy. A) Baseline renal angiography. B) Angiography immediately after β-radiation. Blue arrows mark the length of the radiation source. The radiation source typically was lying against the inferior wall of the renal artery. C) Two-month renal artery angiographic follow-up.
FOLLOW-UP
Animals were followed up at one or two months, at which time they underwent final angiography and intravascular ultrasound of the renal artery, followed by euthanasia. The renal arteries, kidneys, ureters, lungs, liver, spleen, bone marrow and myocardium were excised for histology analysis.
HISTOLOGY
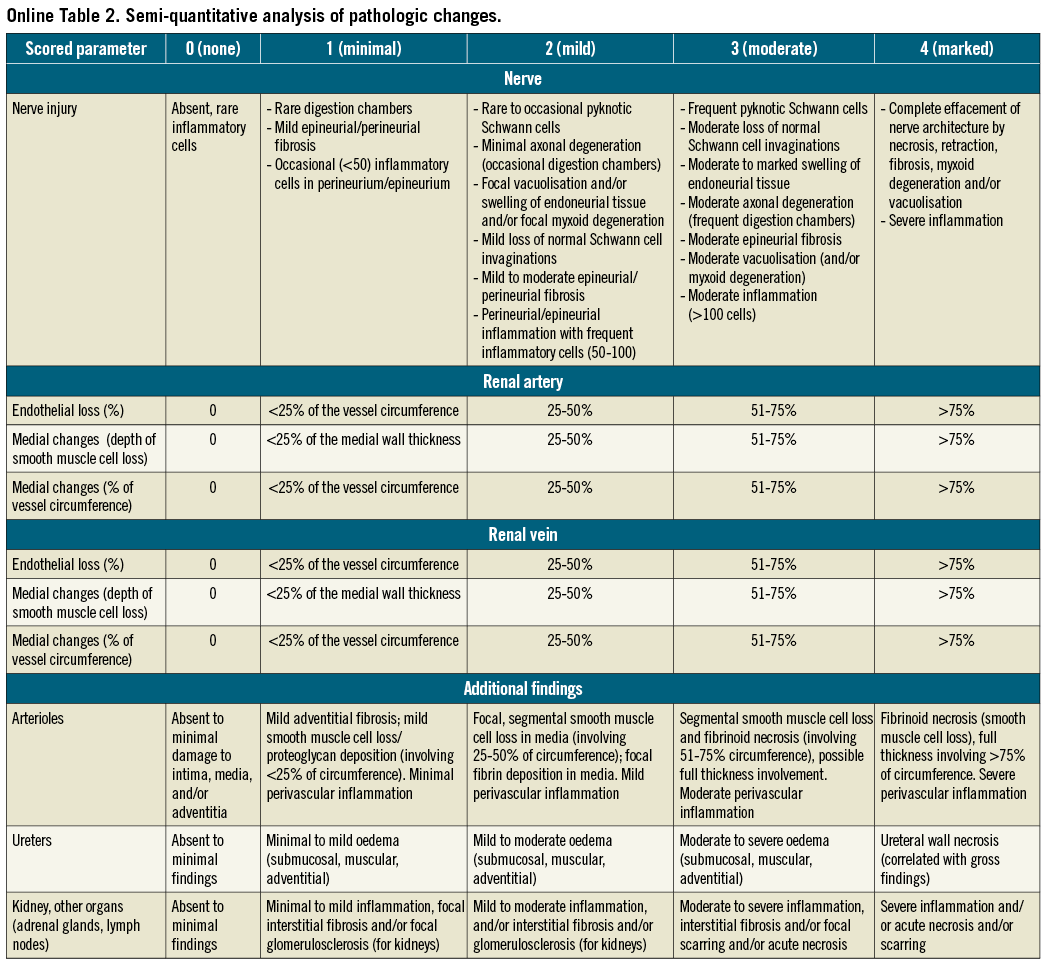
For paraffin processing, each vessel segment was processed through a graded series of alcohols and xylenes. After dehydration, vessels were further cut into two to three segments and sequentially placed on the slide to maintain orientation. Histologic sections were cut on a rotary microtome at 4 to 6 μm, mounted on charged slides, and stained with haematoxylin and eosin and modified Movat pentachrome. Paraffin sections were assessed for injury to the main renal artery and vein and associated branch vessels, nerves, connective tissue, and adjacent lymph nodes utilising the semi-quantitative scoring criteria (Online Table 2). The extent of structural and functional nerve injury was assessed by examination of histologic sections immunostained against S-100 protein for the recognition of axons within nerve fascicles in addition to tyrosine hydroxylase (TH), a functional indicator for norepinephrine synthesis. The intensity and distribution of staining was semi-quantified using a scoring system of 0 to 3: 0=no reaction; 1=patchy/very weak reaction; 2=weak reaction; and 3=strong reaction. A total of 14 representative sections from both treatment groups (25-Gy and 50-Gy) and at one and two-month follow-up were examined and semi-quantified.
The histologic slides of serially sectioned arterial segments were examined systematically from the proximal to the distal end of each renal artery and corresponding renal hilum. Larger segments were further sectioned during embedding. To assess treatment reaction of the vessel, adjacent nerve fascicles, and adjacent organs (kidney, lymph nodes, ureters, and renal veins), ordinal data were collected for multiple parameters, including endothelial loss, arterial and venous medial injury (depth and circumference), inflammation, degenerative changes and necrosis. These parameters were semi-quantified using a scoring system of 0 to 4: 0=none; 1=minimal; 2=mild; 3=moderate; and 4=marked. In slides with >1 artery sections, the section with the most injury was scored.
STATISTICAL ANALYSIS
Statistical analysis was performed using Prism version 5 (GraphPad Software, La Jolla, CA, USA). Numerical parameters with normal Gaussian distributions (according to the Kolmogorov-Smirnov test) are reported as mean±1 standard deviation; other parameters are reported as median with 25th-75th quartiles. Means between groups were compared using Student’s t-test; p-values <0.05 were considered statistically significant.
Results
Five animals received a radiation dose of 25 Gy (two animals had unilateral brachytherapy) and five animals 50 Gy (two animals had unilateral brachytherapy), resulting in a total of eight arteries treated with a dose of 25 Gy and eight arteries with 50 Gy. Four arteries (in four different animals) served as control. Follow-up duration was one month (three animals with 50 Gy, three animals with 25 Gy) or two months (two animals with 50 Gy, two animals with 25 Gy). Renal artery brachytherapy was performed without procedural complications. There was no change in haemodynamics and no acute renal artery damage by angiography. Several animals developed renal artery spasm during guiding catheter cannulation, which was resolved with intra-arterial nitroglycerine.
FOLLOW-UP ANGIOGRAPHY
Follow-up angiography prior to euthanasia at one or two months showed patent renal arteries without stenosis and excellent flow and good kidney opacification. There was no distal vessel occlusion (Figure 1). All animals underwent intravascular ultrasound interrogation which showed smooth renal artery walls without any injuries. Renal artery ostia were normal (Figure 2).
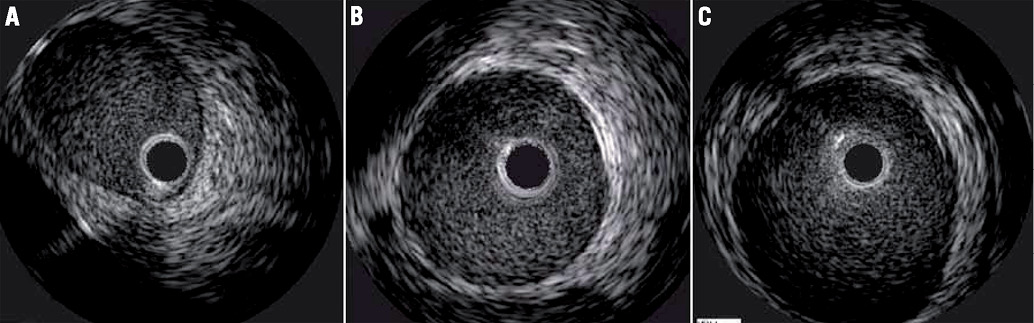
Figure 2. Representative examples (A-C) of intravascular ultrasound at two months after renal artery brachytherapy.
PATHOLOGY EVALUATION OF NERVE DAMAGE
Light microscopic evaluation at one and two months showed varying degrees of thermal injury. Nerve fascicle pathology was described as focal hypocellular fascicles with cellular degeneration and/or by some cells having vacuolated cytoplasm; and/or having mild perineural inflammation with/without fibrosis consistent with a post-injury chronic phase of tissue healing (Figure 3). Quantitatively, at one-month follow-up, 50% of the nerves had some degree of damage (grade ≥1). At two-month follow-up, a dose-related effect was seen: the percentage of damaged nerve fascicles nearly doubled (Figure 4). The degree of injury correlated with radiation dose, with a higher percentage of nerve injury grades two and three among the 50 Gy group (Figure 5).
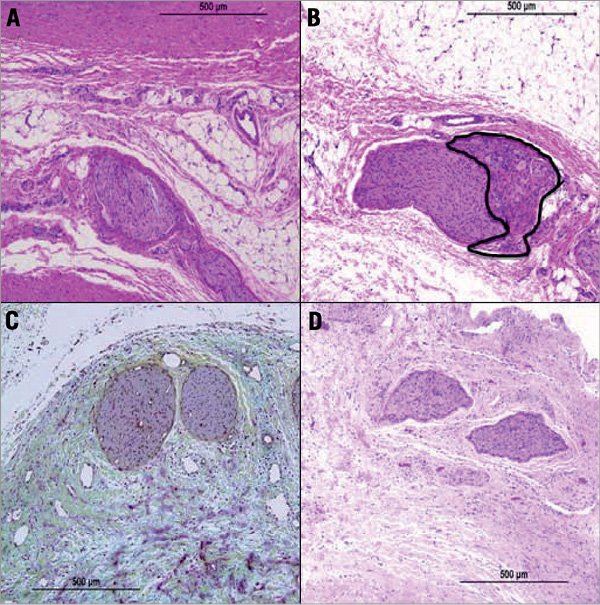
Figure 3. Histological assessment of renal nerve injury. A) High power image showing nerve fascicle with myxoid degeneration, H&E. B) High power image showing nerve fascicles with endoneural/perineural inflammation and cellular degeneration (blue area), H&E. C) Relation of injury to the renal artery. Focal periadventitial fat necrosis with fibrosis and myxoid degeneration, Movat. D) High power image showing hypocellular fascicles with cellular degeneration, Movat.
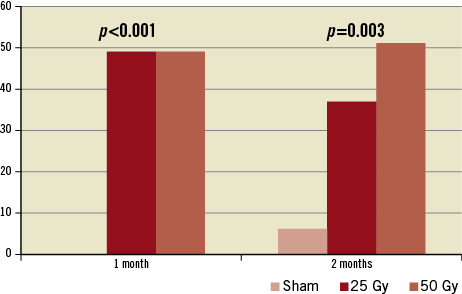
Figure 4. Percentage of damaged renal nerves.
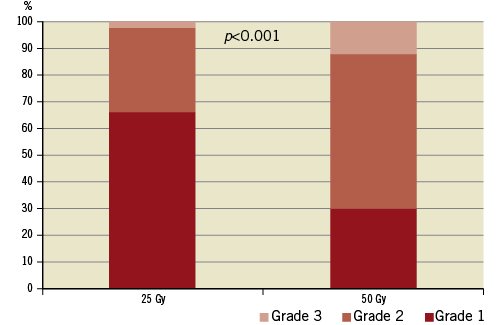
Figure 5. Renal nerve injury grade.
Specific neural staining (Figure 6) for S-100 protein was positive in all examined sections (100% showed grade 3 staining). TH staining (Figure 6) for assessment of norepinephrine synthesis in animals treated with a 25-Gy dose showed grade 3 staining in 5/6 sections (83%) and grade 2 staining in 1/6 (17%). Among animals treated with a 50-Gy dose, 4/8 sections (50%) showed grade 3 staining and 4/8 (50%) showed grade 2 staining for TH. No sections showed grades 1 or 0.
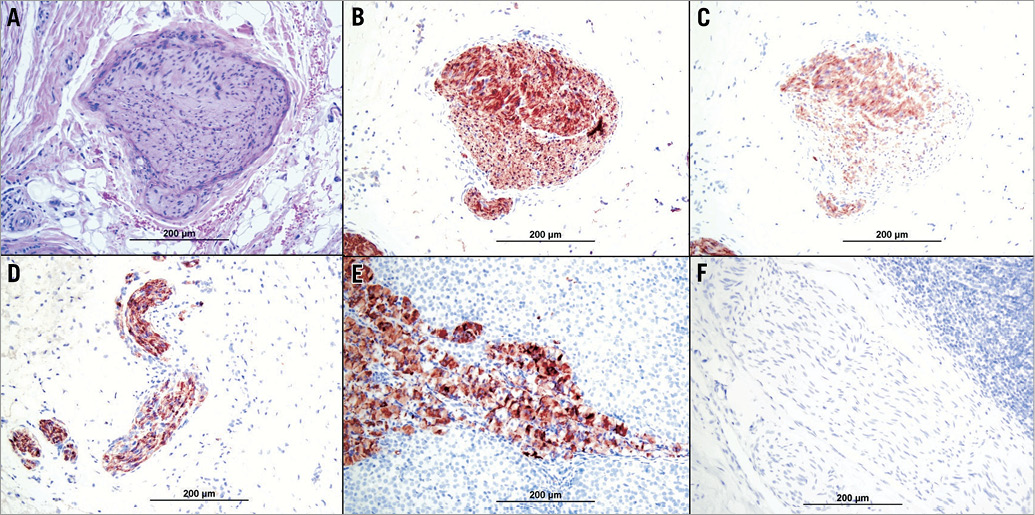
Figure 6. Immunostaining of perivascular nerves. A) Haematoxylin and eosin staining; B) S-100; C) tyrosine hydroxylase; D) S-100 positive control; E) tyrosine hydroxylase positive control; F) S-100 and tyrosine hydroxylase negative control.
There was no damage to the adjacent examined kidney, ureter, adrenals or distant organs (lungs, liver, spleen, bone marrow and myocardium). The surrounding lymph nodes showed non-specific reactive lymphoid hyperplasia.
PATHOLOGY EVALUATION OF ARTERIAL DAMAGE
There was no thrombus or microscopic evidence of stenosis in any of the larger artery sections. There were varying degrees of arteriolar changes in the examined sections, with most showing 2% to 20% endothelial cell loss. Most renal artery sections showed focal adventitial fibrosis with myxoid change. Smaller arterioles within the adventitia and periadventitial soft tissue showed varying degrees of injury, ranging from no injury to minimal perivascular inflammation, typically showing internal elastic lamina and external elastic lamina tear with medial wall fibrinoid necrosis (Figure 7). Overall, large artery and small arteriole injury appeared to be more pronounced in the 50-Gy animals. Pathological evaluation showed no damage to other body tissue.
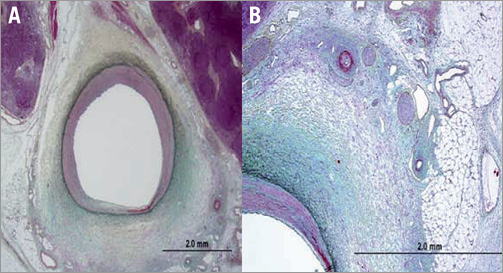
Figure 7. Histological assessment of renal artery injury. A) Image shows relation of injury to the renal artery. Focal periadventitial fat necrosis with myxoid degeneration, Movat. B) High power image showing arteriolar fibrinoid necrosis, periarteriolar chronic inflammation with adventitial fibrosis and adjacent hypocellular fascicles with cellular degeneration and some cells having vacuolated cytoplasm, Movat.
Discussion
This study assessed a novel approach for induction of renal nerve denervation using β-radiation. The preliminary findings of this study indicate that renal nerve denervation using renal artery brachytherapy with β-radiation is feasible and safe. Histological studies have suggested a dose-related effect on renal nerve injury.
The concept of nerve ablation mediated by radiation was introduced for trigeminal neuralgia treatment. Gamma knife radiosurgery is now a standard treatment for trigeminal neuralgia, showing safety and positive long-term results6. Stereotactic radiosurgery causes focal axonal degeneration of the trigeminal nerve. This effect can be identified in contrast-enhanced magnetic resonance imaging scans as contrast agent uptake within the radiosurgical target volume. Radiosurgery can produce necrosis at higher doses7, causing irreversible nerve damage8. In a model of American foxhounds9 treated by 20-75 Gy intraoperative radiation therapy on the sciatic nerves, loss of nerve fibres, particularly large myelinated fibres, was observed. Small myelinated and unmyelinated fibres also demonstrated degenerative changes. No vascular thrombosis or occlusion was observed. In accordance with these findings, our results indicate significant histological damage to surrounding nerve fascicles after both 25-Gy and 50-Gy radiation doses. Immunohistology indicated that, at the time points assessed, the neurons maintained viability with mildly reduced functionality (norepinephrine production). These findings are in accordance with usual radiation effects, which typically take longer periods of time (weeks) to affect the target area. It is anticipated that longer follow-up will show more extensive neuron death and lower norepinephrine production. This pattern is in contrast to the typical effects of radiofrequency ablation, which induce immediate cell necrosis.
The approved clinical indication for brachytherapy involves application of β-radiation in a stented vessel segment for in-stent restenosis treatment. However, the proposed technique of renal nerve denervation using brachytherapy exposes a native vessel to high radiation doses without any prior balloon or stent injury to the treated vessel. While theoretical concerns of damaging the native vessel endothelium, smooth muscle or the elastic lamina layers exist, multiple clinical trials, mostly using beta radiation in coronary arteries, showed the safety of this treatment even in native vessels (i.e., non-stented segments)10-12. The safety of renal artery brachytherapy using beta radiation for renal artery in-stent restenosis was also shown in a few small clinical trials13-15. Given the limited penetrable distance of beta radiation, the finding that the Beta-Cath system was safe in small vessel size (such as the coronary arteries) adds another level of safety as compared to the renal artery, which has a much larger calibre.
Preclinical experience with a beta radiation application at the cavotricuspid isthmus in canines16 provides another indication for the safety in applying this intravascular technique in large vessels/chambers. In this study the cavotricuspid isthmus was irradiated and animals were followed up to six months. Histological assessment of treated canines showed nearly complete sparing of the endocardium with evidence of fibrosis at six months at the level of the myocardium16.
Finally, findings in this study with a follow-up to two months indicate that the lower radiation dose (25 Gy) causes minimal vessel damage, and the higher radiation dose (50 Gy) may cause up to moderate non-transmural damage in a minority of samples with minimal endothelial damage. These findings support the hypothesis that vascular brachytherapy is safe as compared to alternative techniques17.
Limitations
No meaningful physiologic data, such as blood pressure changes, norepinephrine uptake or renal norepinephrine content, could be obtained in this study since the treated animals were non-hypertensive. An additional limitation of the current device iteration is the fact that the radiation source lies along one renal artery wall. An ideal device would be designed to have circumferential contact with the vessel wall in order to target the renal nerves that surround the renal artery. Despite the fact that long-term data suggest no late adverse effects with brachytherapy18, long-term effects and safety of the current approach were not evaluated in the present study since follow-up was designed for two months. Future studies are required to assess the potential long-term effects of renal brachytherapy.
Conclusions
This initial feasibility and safety study of renal nerve denervation mediated by low and intermediate β-radiation dosages indicates that this approach may induce nerve damage while avoiding significant damage to the renal artery. Given these preliminary results, further evaluation is warranted regarding the utility of vascular brachytherapy as an alternative source of energy for renal nerve denervation in the treatment of resistant hypertension.
Conflict of interest statement
The authors have no conflicts of interest to declare.
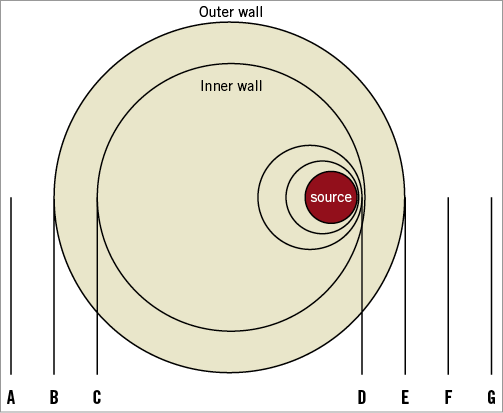
Online Figure 1. Anatomic definitions for Online Table 1 of the inner and outer vessel wall surface distances related to the source.