
Abstract
Aims: The aim of the study was to evaluate the feasibility, safety and efficacy of renal sympathetic denervation with CT-guided periarterial injection of potentially neurolytic agents in pigs.
Methods and results: Unilateral injection of formulations containing either 5M hyperosmolar saline, vincristine, paclitaxel or guanethidine around the renal artery was performed in 24 normotensive pigs with six animals per group. Needle placement and injections were performed under CT fluoroscopy guidance. Blood pressure measurements and CT scans were performed immediately before and after the intervention and four weeks after treatment. After euthanasia, norepinephrine (NE) concentrations of both kidneys were determined. The renal arteries and surrounding tissue were examined histologically to evaluate nerve fibre degeneration. Procedures were technically successful with good periarterial distribution of the injectant in all but one pig in the guanethidine group. No major adverse events or post-interventional complications occurred. In the vincristine group, NE concentrations of the renal parenchyma were lower on the treated side in all pigs with a mean decrease of 53% (38%-62%, p<0.01) compared to the contralateral control. Correspondingly, histological examination revealed neural degeneration in all animals treated with vincristine. In the other groups, no significant drop of NE values, or histological signs of nerve fibre degeneration were found.
Conclusions: CT-guided periarterial injection of the different substances was feasible and safe. Renal sympathetic denervation was achieved with vincristine. In contrast, hyperosmolar saline, paclitaxel and guanethidine do not seem to be appropriate for renal denervation in a pig model at the dosage used.
Introduction
Renal denervation (RDN) by catheter-based transarterial radiofrequency ablation (RFA) of the perirenal sympathetic nerves for the treatment of therapy-resistant hypertension is currently a major topic of research in antihypertensive therapy. First published in a proof-of-principle study in 2009 by Krum et al1 and supported by the results of the subsequent Symplicity HTN-2 trial2,3, the use of this novel method has steadily increased with 15,000 to 20,000 renal denervation procedures performed over the last five years4.
While publication of the HTN-3 trial in 2014 put the efficacy of the procedure into question5, the recently published report of the Global SYMPLICITY Registry showed the method to be effective6.
However, the controversy over the magnitude of the blood pressure-lowering effect of catheter-based RDN shows that there is a need for alternative procedures to RFA. This necessity is supported by the well-known limitations of a catheter-based approach, including anatomical prerequisites, procedural aspects and cost-effectiveness7. A pharmacological extravascular approach might overcome some of these drawbacks, e.g., by avoiding access- and heat-related complications. Recently, several studies have examined chemical RDN as a potential alternative. Perivascular injection of ethanol or vincristine applied transarterially via injection catheter or percutaneously under CT/MRI guidance induced RDN in animal models8-10. Neurolytic effects have also been described for hyperosmolar saline, paclitaxel and guanethidine11-13.
In this study we aimed to evaluate the feasibility, safety and efficacy of CT-guided percutaneous periarterial administration of hyperosmolar saline, vincristine, paclitaxel and guanethidine for renal sympathicolysis in pigs.
Methods
ANIMAL STUDY
The study was approved by the Animal Research Committee and conducted in accordance with regulatory guidelines for the care of laboratory animals in agreement with Directive 2010/63/EU of the European Parliament.
The domestic swine model was chosen for its renal anatomy as arterial diameter and morphology are similar to those of humans14. Twenty-four normotensive domestic pigs (mean weight at baseline: 24.7±2.4 kg, and at four-week follow-up: 33.2±5.3 kg) were divided into four groups with six pigs for each substance: (I) hyperosmolar saline, (II) vincristine, (III) paclitaxel and (IV) guanethidine. Computed tomography (CT)-guided percutaneous periarterial injections were performed unilaterally in each pig, with the contralateral kidney serving as control. Treatment efficacy was assessed four weeks after intervention by determination of the renal norepinephrine (NE) concentrations and histological changes of the renal sympathetic nerve fibres.
INTERVENTIONAL PROCEDURE
All interventions were performed with the animals under general anaesthesia and continuous monitoring of vital signs. Injections were performed using CT fluoroscopic guidance (iFluoro CT, 64-MSCT, SOMATOM® Definition AS; Siemens, Erlangen, Germany). After prone positioning of the pigs, a biphasic CT scan in arterial and venous contrast phases was acquired (XENETIX® 350 [50 ml]; Guerbet, Villepinte, France; arterial phase 15 s/venous phase 40 s delay; 30 ml saline; flow rate 2.5 ml/s) for treatment planning and determination of the puncture site.
Next, ca. 10 ml of local anaesthetic (Lidoject® 1%; Hexal AG, Holzkirchen, Germany) was administered subcutaneously at the entry point. A 20 G spinal needle (Becton Dickinson S.A., Franklin Lakes, NJ, USA) was inserted from a posterolateral angle and advanced to a position directly adjacent to the origin of the renal artery. After aspiration, and administration of 1 ml bupivacaine contrast agent solution (Carbostesin® 0.5%; AstraZeneca, London, United Kingdom/Accupaque™ 240; GE Healthcare, Buckinghamshire, United Kingdom) to ensure optimal needle placement and injectant distribution, the treatment substance was applied. Distribution was monitored by intermittent CT fluoroscopy during injection (iFluoro CT; Siemens). Ten minutes after complete injection, an unenhanced spiral scan was acquired to visualise injectant distribution.
The active substances used for denervation were delivered by the hospital’s in-house pharmacy. The final injection solutions were mixed by the interventional team immediately prior to administration and contained 10 ml of each of the following:
I Hyperosmolar saline 5 mol/l (M) (made by the pharmacy) and contrast agent (Accupaque 240) in a ratio of 9:1.
II Vincristine 0.01 mg/ml (made by the pharmacy) in isotonic saline 0.9%, bupivacaine and contrast agent in a ratio of 7:2:1.
III Paclitaxel (Taxomedac®; Medac GmbH, Hamburg, Germany), bupivacaine and contrast agent in a ratio of 7:2:1.
Taxomedac contains 6 mg/ml paclitaxel and ethanol 395 mg/ml with 527 mg/ml macrogolglycerolricinoleat (Cremophor) as solutiser.
IV Guanethidine monosulphate (10 mg/ml) (Sovereign Medical, Essex, United Kingdom) and contrast agent in a ratio of 9:1.
Detailed information is given in Table 1.
ASSESSMENT OF TECHNICAL OUTCOME
The post-interventional native CT scans were assessed in consensus by two experienced readers.
Procedures were defined as technically successful if the injectant was surrounding the renal artery from origin to mid segment circumferentially. Distribution of the injectant was graded on a three-point scale: 1 (insufficient), 2 (good), and 3 (excellent) (Table 2). Procedure time was defined as the interval between the pre-interventional and the post-interventional CT scan.
ASSESSMENT OF SAFETY AND EFFICACY
At four-week follow-up, a biphasic CT scan was performed to identify potential complications such as renal artery stenosis/occlusion, hydronephrosis, infarction, or other inadvertent damage to the adjacent tissues. Immediately after the CT scan, the animals were euthanised in deep anaesthesia. Gross examination of the renal parenchyma, adrenal glands and periarterial connective tissue stroma containing the ureter, renal vein and renal lymph nodes was performed to search for effects such as thromboembolism, infarction, necrosis and inflammation.
For histological analysis after nephrectomy, a tissue block including the renal vessels and associated soft tissues was dissected and immersed in 4% neutral buffered formalin. The renal artery and periarterial stroma containing the renal nerves were sectioned transversely at 3-4 mm intervals. Tissue samples were then sectioned into 5 μm slices and stained with haematoxylin and eosin (H&E) and with Elastica van Gieson (EvG) stain. In addition, immunostains against S-100 protein and neurofilament protein were prepared. An independent, experienced neuropathologist examined all histologic slides looking for evidence of injury to the renal arteries, and to the periarterial renal connective tissue containing the renal nerves. Nerve injury was graded qualitatively according to a four-point Likert scale with fibrosis as the main criterion for successful denervation modified to the scale by Sakakura et al (Table 3). In addition, the periarterial tissue was divided into four quadrants to assess the circumferential effect.
The NE concentrations in the renal parenchyma were determined to assess the effectiveness of renal sympathicolysis. Immediately after explantation, both kidneys were homogenised in 0.1% formic acid and centrifuged. NE measurement was performed following liquid extraction by high-performance liquid chromatography (HPLC) according to Bauch et al15. NE content is expressed as ng/g kidney. NE in the contralateral untreated kidney served as control.
Blood pressure (BP) measurements in mmHg were performed in all animals immediately before (baseline) and directly after the intervention and at the four-week follow-up.
Statistical comparisons were made using paired t-tests (SPSS Statistics, Version 21; IBM Corp., Armonk, NY, USA); p<0.05 was considered statistically significant.
Results
TECHNICAL OUTCOME
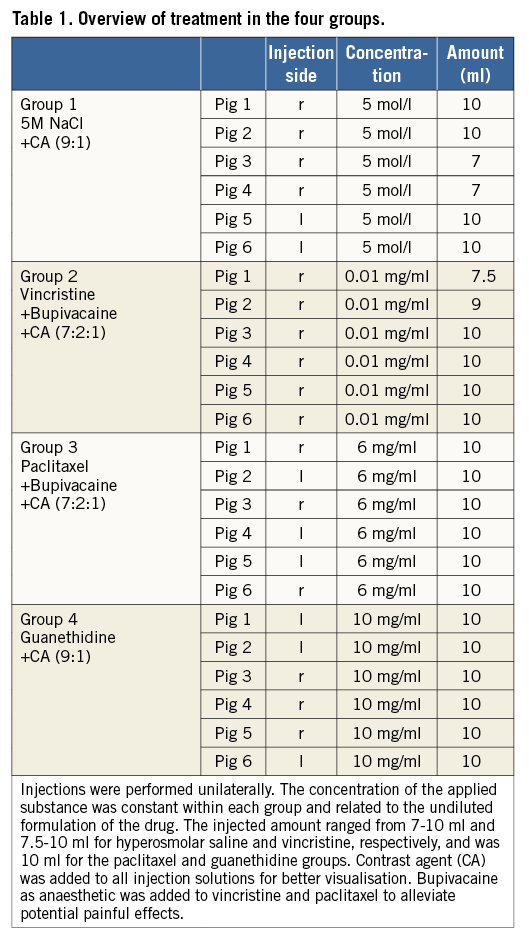
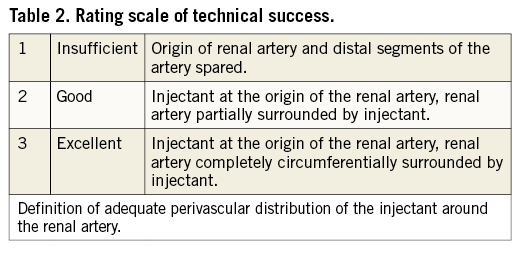
All but one intervention were considered technically successful with good to excellent periarterial distribution of the injectant (2.6±0.6) (Figure 1). In one pig in the guanethidine group, the substance was injected below the renal artery since a lower aberrant pole artery was mistaken for the target vessel (Figure 2). Injections were performed on the right side in 16 and on the left side in eight animals. There were no significant differences between the groups. Mean procedure time was 27 (±15) min (median 23 min).
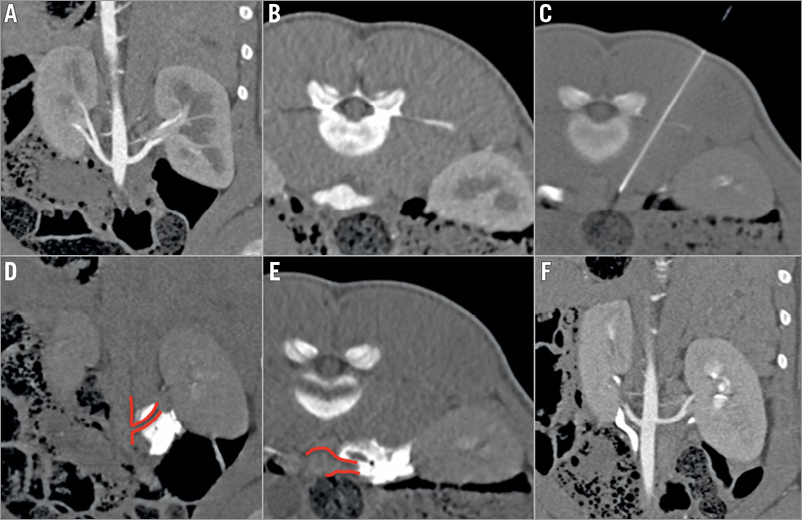
Figure 1. CT-guided procedure and control. A) & B) Pre-interventional CTA (coronal and axial views). Single artery supply of both kidneys with visualisation of the origins and proximal segments. C) CT-guided needle placement adjacent to the origin of the left renal artery. D) & E) Unenhanced control scans immediately after injection for visualisation of distribution of the injectant with good coverage of the artery (score 3). F) CTA after four weeks shows normal renal arteries, kidneys and ureters without any sign of procedure-related early complications.
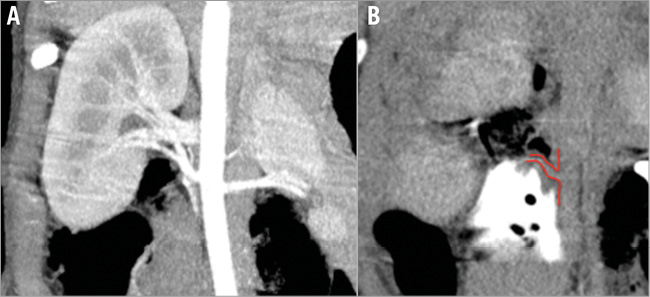
Figure 2. Example illustrating insufficient injectant distribution. A) Pre-interventional CTA with visualisation of the renal arteries (coronal MIP). B) Insufficient distribution (score 1) of the injectant which lies below the right main renal artery (pig 4 of the guanethidine group). A lower aberrant renal artery (not visible in panel A) was mistaken for the main renal artery.
SAFETY
Clinical adverse events did not occur during the interventions or follow-up. The four-week CT scans showed no signs of arterial or ureteral stenosis or other treatment-related abnormalities.
GROSS EXAMINATION AND HISTOPATHOLOGIC ASSESSMENT
In none of the animals did gross examination of the treated kidneys and surrounding structures reveal any adverse effects, such as renal artery stenosis, kidney infarction or hydronephrosis.
Histopathological examination revealed circumferential neural damage in all vincristine samples with damaged nerve fascicles in two to three quadrants (mean 2.7) with an emphasis on the dorsal side of the renal artery. Neural injury was found at distances of 0.2 to 7.1 mm from the arterial lumen and corresponded to grade 1 to 3 findings with perineural and endoneural fibrosis compared with the untreated control (grade 0, Figure 3A). Changes were visualised best with the EvG stain (Figure 3B, Figure 3C). Perineural fibrosis as well as mild vacuolisation was also seen in the H&E stains (Figure 3D), yet more discreet compared to EvG and earlier studies with ethanol. Immunostains against S-100 protein and neurofilament protein also revealed neural damage within the vincristine-treated samples (Figure 3E-Figure 3H).
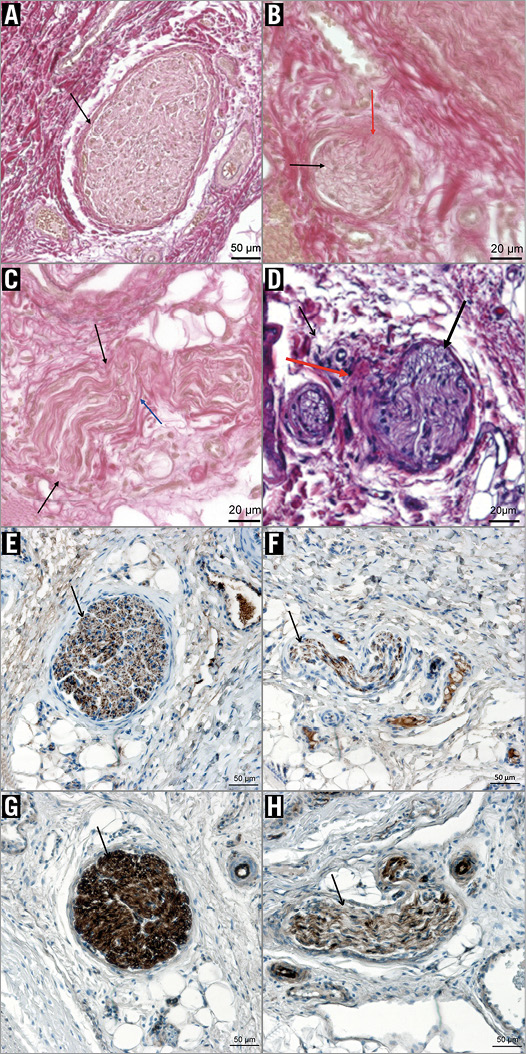
Figure 3. Histologic samples of pig 3 of the vincristine group. A) Untreated control (grade 0) with good differentiation of the collagen-rich connective tissue and the periarterial nerve fascicle. The latter shows a thin perineurium (black arrow) and normal axonal structures. (EvG stain, 10x magnification). B) Treated side (grade 1): damaged nerve fascicle (black arrow) with diffuse thickening of the perineurium and fibrotic strands extending into the fascicle (red arrow). C) Treated side (grade 2): impaired differentiation of the thickened perineurium (black arrows). There is also fibrosis within the fascicle (blue arrow). (EvG stain, 20x magnification). D) H&E stain (20x magnification) of treated side (grade 1-2): epineurium/perineurium shows mild to moderate inflammation (red arrow). Endoneurium: mild vacuolisation is seen (black arrow). E) - H) Immunostains against neurofilament protein (E & F) and S-100 protein (G & H) with comparison of healthy (E/G) and degenerated (F/H) fascicles (10x magnification): neurofilaments (brown points) appear rarefied and less organised within the damaged fascicle. In concordance to this, less S-100 protein is stained in the damaged fascicle, correlating to a rarefying of Schwann cells.
In contrast, no histological signs of neurolysis were found in any of the pigs treated with the other three solutions investigated. Damage to the vessel walls or the surrounding tissues including the ureters was not found in any of the four groups.
NOREPINEPHRINE MEASUREMENTS
In the vincristine group, NE concentration was lower on the treated side in all pigs with a mean drop of 53±9% (min/max. 38%-62%, p<0.01).
A relationship between the amount of the injected vincristine volume, distribution score, NE decrease and degree of degenerated nerve fascicles was not found.
Hyperosmolar saline, paclitaxel and guanethidine did not lead to a significant NE decrease of the treated kidneys with mean changes of +18% (p=0.73), +1.5% (p=0.96) and -4.75% (p=0.67), respectively. In some animals of all three groups, the NE value was even higher on the treated side (Table 4, Figure 4).
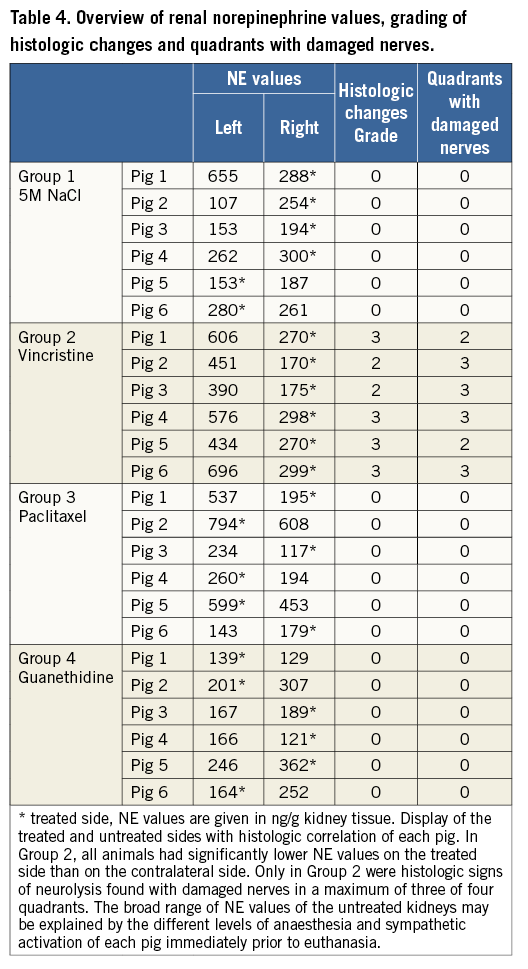
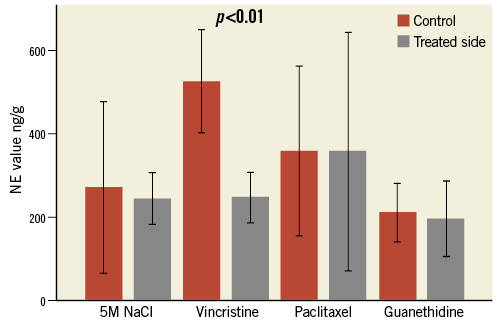
Figure 4. Norepinephrine (NE) values. Comparison of mean NE value of the treated (grey columns) and untreated (red columns) kidneys in all four substance groups (ng/g kidney tissue) (error bar represents 95% CI). In the vincristine group, the mean NE value of the treated side is significantly lower than that of the control side (–53%). In the other three groups, there was no significant difference between the treated and untreated sides.
BLOOD PRESSURE MEASUREMENTS
There was no significant difference in systolic and diastolic BP at baseline and at four-week follow-up, either among the animals within each of the four groups, or between the groups.
Mean BP in all groups was 106/43 (±21/12) mmHg before intervention, 111/49 (±21/14) mmHg immediately after the procedure, and 105/50 (±19/12) mmHg after four weeks (Figure 5).
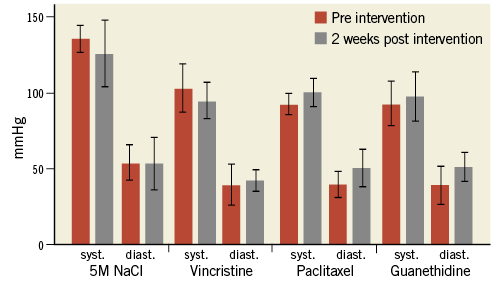
Figure 5. Blood pressure. Mean blood pressure values in all four substance groups before (red columns) and four weeks after the intervention (grey columns) without significant differences between baseline and post-interventional values (error bar represents 95% CI).
Discussion
In the search for alternatives to RDN via catheter-based RFA, recent studies have shown the feasibility of pharmacologic RDN by administration of neurolytic substances in animal models and in first clinical applications8,9,16-18.
In this study, successful RDN with corresponding histopathological changes and a decrease in NE of the treated kidney were achieved in all animals of the vincristine group. Our results correspond to those of Stefanadis et al, who achieved RDN in swine and in a first patient by transarterial administration of 0.1 mg vincristine using a microperforated balloon catheter16,17.
The 53% lower NE concentration in the treated kidneys compared to the contralateral untreated control kidneys in our study is comparable to reported NE reductions achieved with other methods, e.g., 47% by RFA in humans, 50% by intravascular ultrasound in pigs and 53% by periarterial ethanol injection in pigs1,8,9,19. Yet, a direct comparison of the results is limited since the method of NE measurement and the selected reference values differed between the studies. Although unlikely, a treatment-related increase of NE in the contralateral untreated kidneys cannot be completely excluded.
Although neurolytic effects with hyperosmolar saline, paclitaxel and guanethidine were achieved in earlier studies20-23, in this study periarterial injection of these substances did not result in RDN. One reason could be an unfavourable periarterial distribution of the injectant attributable to the dorsal approach. In both pigs and humans, the maximum average number of sympathetic nerves is found in the proximal and middle segments of the renal artery with a higher density on the ventral side24. Immediately after injection, the largest amount of the injectant was located posterior to the renal artery with the anterior parts of the vessel being underexposed or unexposed. Although histologic examination revealed a circumferential nerve degeneration in the vincristine samples, the effect was pronounced on the dorsal side of the artery. With good distribution scores in all groups ten minutes after the injections, the results indicate that the initial pattern of injectant deposition may be of particular importance. At a distance of ~3 mm from the aorta, nerves are located at radial depths of up to 8 mm25. With degenerated nerves found at distances of up to 7.1 mm from the arterial lumen, the percutaneous extravascular approach potentially affects nerves that are out of reach for current intravascular RF RDN systems which achieve ablation depths of 3 to 4 mm.
With ongoing dispersion of the substance into the adjacent tissue, a decrease of the substance’s concentration at the target region may be supposed. Moreover, diffusion is influenced by the hydrophilic properties of the injectant and the osmotic gradient between injectant and surrounding tissue. Hyperosmolar saline diffusion into the adjacent connective tissue was probably fast with limited effect on the sympathetic nerves. Differences in the neurotoxic potential between the agents used due to different modes of action may be another major contributing factor. The neurotoxic effect of vincristine and paclitaxel is based on a dysfunction of the axoplasmic transport, which is crucial for cellular function. Although addressing the same intracellular structures, the substances differ extensively in efficacy and neurotoxic potential, which is far higher for vincristine26,27. The neurolytic effect of guanethidine and hyperosmolar saline is based on blocking excitatory vesicular release by replacing norepinephrine in synaptic vesicles or by induction of electrolyte shifts in the axons.
Since ours was the first study using these four substances for RDN after percutaneous periarterial administration, data on dosage and concentrations required to achieve RDN did not exist. Therefore, doses were calculated according to the pigs’ body weight and on the basis of concentrations administered in earlier studies and common doses in systemic delivery.
Successful RDN by transarterial administration of 0.15 to 0.6 mg ethanol via microinjection catheter was reported by Fischell et al8.
Streitparth et al used a 12 to 47 times higher dose (10 ml ethanol) compared to Fischell et al to achieve the same NE decrease (53% vs. 54%) by using an image-guided percutaneous technique9. On the other hand, RDN was successfully achieved with vincristine despite a lower concentration (0.01 mg vincristine/ml isotonic saline) compared to Stefanadis et al using an endovascular approach (0.025 mg vincristine/ml isotonic saline)10,16.
Obviously, a direct dose transfer between differing experimental settings with different ways of substance administration and animal models is difficult. In consequence, method-specific doses and concentrations need to be established for each approach and injection method in future studies.
An interaction between contrast media and the drugs used may be considered. Yet, due to the small portion of added contrast agent and the results of earlier studies we did not assume a negative impact on efficacy9.
Corresponding to other renal denervation studies in pigs10, a drop in BP was not found in any of the treated pigs. Since RDN in a therapeutic setting in patients is performed bilaterally, a significant effect on BP after a unilateral treatment in healthy juvenile normotensive animals was not expected.
Extravasation of vincristine and paclitaxel may cause severe tissue necrosis9,28. The dose of 0.07 mg vincristine used in this study was far lower than the commonly used doses for chemotherapy. This may explain why we found no post-procedural complications of the surrounding perirenal structures such as renal artery stenosis or ureter stenosis with consecutive hydronephrosis in any of the pigs.
Study limitations
Sympathetic nerve activity is difficult to measure. Determination of the NE concentration in renal parenchyma was shown to be a meaningful parameter in earlier studies. However, renal NE values may potentially be influenced by the different stress levels of the animals and depth of anaesthesia. Furthermore, NE values may differ slightly between two normal kidneys as a series of untreated pigs by our group has shown (unpublished data). This biometric variance may explain higher NE values on the treated side in some of the pigs of this study. This may even obscure treatment-related effects.
A quantitative assessment of sympathicolysis by counting an absolute amount of intact and degenerated nerves in the histologic samples was not performed. Since the number of visible nerves was highly variable, even between adjacent samples of one artery, a ratio of intact and degenerated nerves did not seem to be a stable parameter for successful denervation. Additionally, a morphologically intact nerve may be inoperable and vice versa. This may also explain the lack of correlation between histological nerve injury and functional NE reduction in some animals.
An enzyme histochemical analysis of tyrosine hydroxylase activity and therefore NE synthesis was not performed since we worked with formalin-fixated specimens. However, immunostains against S-100 and neurofilament protein together with H&E and EvG stains provided clear results. An examination of the dose-dependent efficacy of the substances investigated or of the clinical parameters of renal function such as creatinine and glomerular filtration rate was not performed and should be part of future studies.
Conclusions
The results of this study show that RDN by CT-guided percutaneous vincristine administration is feasible, effective and safe. CT guidance allows a precise needle placement and subsequent injection. Guanethidine, hyperosmolar saline and paclitaxel failed to induce renal denervation.
| Impact on daily practice Catheter-based RFA for renal denervation is limited by anatomical and procedural restraints and furthermore shows a highly variable outcome. CT-guided chemical RDN with neurolytic substances such as vincristine is effective and safe and may be an alternative to RFA in patients who are not eligible for a catheter-based approach. |
Acknowledgements
We thank U. Bernhardt, InnoRa GmbH, for laboratory analyses.
Conflict of interest statement
The authors have no conflicts of interest to declare.

