
Abstract
Aims: Renal denervation using the point-by-point application of radiofrequency energy delivered by the first-generation Symplicity system is effective in lowering office blood pressure but may be time-consuming. The OneShot™ Renal Denervation System with a balloon-mounted spiral electrode potentially shortens and simplifies the procedure. This study is a hypothesis-generating first-in-human study to assess feasibility, and to provide preliminary efficacy and safety data.
Methods and results: Eligible patients had a baseline office systolic blood pressure ≥160 mmHg (or ≥150 mmHg for diabetics) and were on two or more antihypertensive medications. Nine patients were enrolled. The primary endpoint, the insertion of the OneShot balloon into each renal artery and the delivery of radiofrequency energy, was achieved in 8/9 (89%) of patients. The one failure (the first patient) was due to generator high-impedance safety shut-off threshold set too low for humans. Adverse events were minor. No patient developed renal artery stenosis. Baseline BP was 185.67±18.7 mmHg and the reductions at 1, 3, 6 and 12 months were 30.1±13.6 (p=0.0004), 34.2±20.2 (p=0.002), 33.6±32.2 (p=0.021) and 30.6±22.0 (p=0.019).
Conclusions: The OneShot renal denervation system successfully delivered radiofrequency energy to the renal arteries in a short and straightforward procedure. Australian New Zealand Clinical Trials Registry - URL: anzctr.org.au. Trial identification: ACTRN12611000987965.
Introduction
Percutaneous transcatheter renal sympathetic denervation (RDN) is a promising treatment for refractory hypertension (HT). RDN was found in one series of clinical studies to reduce office systolic blood pressure (BP) by as much as a mean of 30 mmHg with 85% of subjects experiencing sustained reductions of 10 mmHg or more out to two years after RDN1-4.This degree of BP reduction may reduce stroke and myocardial infarction rates and is anticipated to translate into improved life expectancy6-8. Lowering BP by RDN has been shown to improve glycaemic control and reverse left ventricular hypertrophy7-9. Furthermore, improvements to renal function, sleep apnoea and heart failure are suggested7-9.
The first-generation percutaneous device (Symplicity; Medtronic, Santa Rosa, CA, USA) achieves RDN by ablating sympathetic nerves that run in the renal artery adventitia by application of radiofrequency (RF) energy in a distal-to-proximal, point-by-point spiral pattern. This typically requires four or more ablations, each of two minutes duration, to each renal artery.
The OneShot™ Renal Denervation System (Covidien, Campbell, CA, USA) is an irrigated RF balloon designed to deliver energy to achieve denervation of renal arteries using a single two-minute ablation to each renal artery10. We report on outcomes in patients enrolled in the Renal Hypertension Ablation System (RHAS) trial who were treated using the OneShot system.
The aims of this first-in-human study were to provide hypothesis-generating safety and feasibility data concerning the OneShot renal denervation device.
Methods
STUDY DESIGN
The RHAS study was a prospective, single-centre, non-randomised feasibility study to evaluate the safety and effectiveness of the OneShot Renal Ablation System for the treatment of chronic resistant hypertension. The nine patients enrolled in this trial at Mercy Angiography, Auckland, New Zealand, were followed out to 12 months. The study was approved by the New Zealand Ethics Committee and all patients gave written informed consent.
STUDY DEVICE
The major components of the OneShot System are the irrigated RF balloon catheter and a radiofrequency generator (RFG) with an integrated pump (Figure 1)10. A monopolar silver electrode mounted on the non-compliant balloon in a helical configuration (Figure 1A) delivers RF energy to the arterial wall, ensuring delivery in a spiral pattern without the need to manipulate the catheter. The balloon is delivered to the renal artery over a conventional 0.014 inch interventional guidewire through a guide catheter. Radiopaque markers identify the balloon ends for positioning under fluoroscopy (Figure 1). Balloon catheters are available in 5, 6 and 7 mm diameters. The guide catheter size used for this trial was 9 Fr but the current balloons are compatible with 7 and 8 Fr guides. The balloon is inflated to nominal size at 1 atmosphere pressure with normal saline delivered by the pump integrated to the RFG. The inflated balloon stabilises electrode contact with the renal arterial wall. Renal angiography, showing occlusion of the artery by the balloon, ensures contact of the ablating spiral electrode with the arterial wall (Figure 1D). During ablation, saline seeps from the eight irrigation holes that are present in the balloon close to the electrode (Figure 1E). These are designed to mitigate damage to non-target arterial tissue, effectively allowing the energy to penetrate deeper into the tissue. The spiral monopolar electrode in conjunction with an external dispersive electrode delivers a single, two-minute RF ablation to each artery, and in long arteries several applications of RF energy are possible.
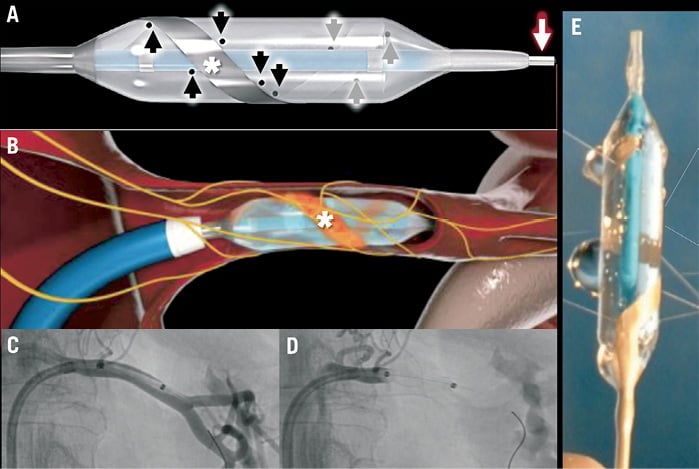
Figure 1. The OneShot Renal Denervation System. (A) The OneShot balloon with the spiral electrode indicated by the asterisks. The black arrows indicate the eight irrigation holes in the balloon adjacent to the electrode. The balloon is delivered over a 0.014 inch wire (white arrow). The diagram in (B) is a left renal artery cutaway showing the inflated balloon, with the spiral electrode in an orange colour. The sympathetic nerves are shown in yellow. Shown in (C) is a left renal selective angiogram showing the position of the OneShot in this artery. In (D), the balloon is inflated and occludes the renal artery, indicating the likelihood of apposition of the electrode to the arterial wall. (E) shows saline irrigation from the balloon holes.
The RFG features a touch screen interface for user input and displays instructional messages and procedural feedback such as power delivered, impedance, and remaining treatment time. The RFG displays warning messages and triggers automatic shut-offs to ensure safe operation of the system.
Patients
The inclusion and exclusion criteria were similar to those of the Symplicity HTN trials, although patients with two rather than three antihypertensive agents could be enrolled1. Patients 18 years and older were suitable for enrolment if they had a consistent office-measured systolic BP greater than or equal to 160 mmHg (or greater than 150 mmHg for patients with type 2 diabetes) despite treatment with two or more antihypertensive medications, had renal artery diameters between 4 and 7 mm and a segment of artery at least 20 mm in length devoid of stenosis. Patients were excluded if they had a glomerular filtration rate less than 45 mL/min/1.73 m2. Before ablation, all patients underwent computed tomography angiography (CTA) to exclude those with inappropriate renal artery diameters, more than mild renal artery stenosis, and serious renal abnormalities.
Follow-up
Patient assessments were carried out before the procedure and at follow-up at 1, 3, 6 and 12 months. At each follow-up office blood pressure was measured, medications reviewed and potential adverse events assessed.
Endpoints
The primary endpoint was the successful insertion and positioning of the OneShot balloon in each renal artery and delivery of low level radiofrequency energy. Secondary endpoints included: 1) acute procedural safety, defined as absence of serious groin complications or vascular access site complications; 2) procedural success, defined as freedom from complications associated with the delivery and/or use of the OneShot device or the procedure; and 3) blood pressure lowering effects of the procedure measured by the reduction of office systolic blood pressure at six months compared with baseline.
Study management
Data were monitored to ensure compliance with the protocol and to verify that the data matched source medical records. An independent clinical events committee adjudicated study endpoints and events that occurred throughout the study. The data and safety monitoring board reviewed all safety issues.
Statistical analysis
This study was hypothesis-generating and was designed to provide initial performance information for the OneShot device. The sample size was not defined on the basis of an endpoint hypothesis. Descriptive statistics were provided for baseline demographics, procedural characteristics and study outcomes. Continuous variables are expressed as mean (standard deviation) and discrete variables as percentages. Changes in BP at follow-up visits from baseline were evaluated using the paired t-test. A p-value of <0.05 was defined as statistical significance; corrections for multiple comparisons were not made because of the hypothesis-generating nature of the trial. All statistical analyses were performed using Statistical Analysis System (SAS) for Windows version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline demographics and clinical characteristics for the nine patients are shown in Table 1. Procedural variables are summarised in Table 2.
Minor adverse events to six months were mostly periprocedural and were haematoma or bruising (4), vomiting (1), headache (1), flushed feeling (1), exacerbation of gout (1), brief coolness and numbness of one foot (1) and a vasovagal episode with brief loss of consciousness (1).
The primary study endpoint, which was the ability to insert the OneShot balloon in each renal artery and to deliver low level radiofrequency energy, was achieved in 8/9 (89%) of patients. The one failure was with the first patient when the RF generator high-impedance safety shut-off threshold was set too low for humans. After minor reprogramming there were no further issues.
Procedural time was short, with the time from balloon insertion to the end of the procedure a median of 17 minutes.
At follow-up after renal denervation mean office systolic BP (Table 3, Figure 2) significantly reduced from 185.67±18.7 mmHg to 155.58±18.84, 151.46±19.93, 152.08±22.27, and 155.89±27.27 mmHg at 1, 3, 6 and 12 months, respectively (p=0.0004, 0.002, 0.021 and 0.019 at 1, 3, 6 and 12 months). At one month, 7/8 (87.5%) patients had a mean reduction of >10 mmHg in office systolic BP, and at 3, 6 and 12 months the response rate was 7/8 (87.5%), 6/8 (75.0%) and 5/6 (83.3%), respectively.
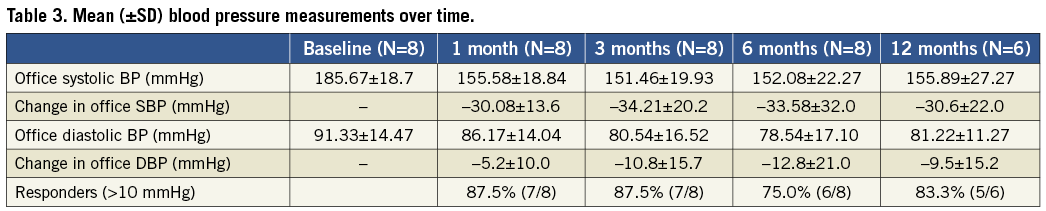
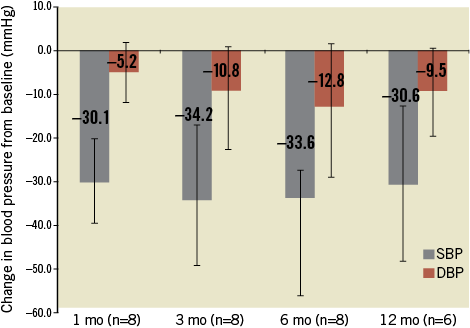
Figure 2. Change in mean (with SD) office blood pressure at 1, 3, 6 and 12 months. Error bars are 95% confidence intervals. The numbers in parenthesis indicate the number of patients with data at each visit.
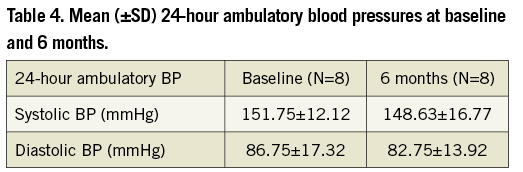
While 24-hour ambulatory BP was not a predefined endpoint in our trial, the systolic BP reduction (Table 4) was directionally similar but less in magnitude than the reduction in office BP (Figure 2). Twenty-four hour ambulatory blood pressure showed a mean decrease of 3 mmHg for systolic BP and a 4 mmHg reduction in diastolic BP.
Six-month CTA in the eight treated patients showed no significant change since baseline and in particular there was no occurrence of renal artery stenosis.
In the eight patients there was no change in renal function assessed by eGFR and/or serum creatinine between baseline and 6 and 12 months.
Discussion
The main findings of this study are:
1. The OneShot irrigated radiofrequency balloon with a spiral monopolar electrode was successfully inserted and positioned in each renal artery and delivered radiofrequency energy in eight of nine patients. The one failure was with the first patient and was due to the high-impedance safety shut-off threshold set too low for humans. This was subsequently solved by minor generator reprogramming.
2. The device was safe with only minor adverse events at six months post procedure.
3. The device was effective with over 75% of patients having a reduction of systolic BP of >10 mmHg. The mean reduction at 12 months was 30 mmHg.
4. No renal artery stenosis was detected by CTA at six months.
The OneShot balloon stabilises the system in the artery for the single delivery of energy by the spiral electrode and the operator does not have to redirect the ablation catheter for multiple point-by-point ablations. Hence, delivery of radiofrequency energy with the OneShot should be more predictable and consistent. The irrigated monopolar design has evenly spaced holes that deliver saline throughout the procedure. Active cooling maintains safe electrode-tissue temperature, reduces the chance of thrombus and char, and limits the damage to the endothelium11. The first-generation Symplicity device requires four or more ablations on each side resulting in at least 16 minutes or more of ablation; in contrast, the OneShot system delivers RF energy on each side for two minutes, which should result in shorter procedural times and shorter duration of painful stimuli.
Although our patient numbers are small, the magnitude of office systolic BP reduction in the eight treated patients (mean office systolic and diastolic reductions of 34 and 13 mmHg, respectively) at six months is similar to that with treated patients in the Symplicity HTN-2 trial (mean office systolic and diastolic reductions of 31 and 12 mmHg, respectively)1. In the current trial the reduction in office BP compared with baseline is sustained at 12 months. One patient did not have 12-month follow-up because she was unwell with chemotherapy for recently diagnosed breast cancer, and the other was in hospital following stent implantation subsequently complicated by stent thrombosis associated with clopidogrel resistance.
The procedure was safe with minor adverse events to 12 months, and with no change in renal function and no appearance of renal artery stenosis. There is preclinical evidence that the presence of irrigation during treatment, while mitigating damage to surrounding non-target tissue, allows energy to be effectively transferred deeper into the tissue.
The 24-hour ambulatory BP reduction in this trial was not as great as we expected. However, it was not a predefined endpoint and our patient numbers are small. In the Symplicity HTN-2 trial1, only about 40% of patients had 24-hour ambulatory blood pressure monitoring at baseline and at six months, and the mean decrease in BP in the treated group was 11/7 mmHg compared with 33/11 in office BP1. It has been suggested that the less pronounced effect on 24-hour ambulatory BP may be related to greater activation of the sympathetic nervous system with office BP measurement than with ambulatory BP monitoring, hence the more marked effect after renal denervation12. Office BP measurement is widely used in hypertension studies and is of prognostic importance13. The relatively small change in 24-hour ambulatory BP could be because some of the patients had “white-coat” hypertension.
Limitations
This is a small safety and hypothesis-generating first-in-human trial that is not randomised or blinded.
Summary
This first-in-human use of the OneShot renal denervation system successfully delivered RF energy to the renal arteries in a short and straightforward procedure.
Funding
The trial was funded by Maya Medical, now Covidien (Campbell, CA, USA).
Conflict of interest statement
J. Ormiston was a minor shareholder in Covidien. The other authors have no conflicts of interest to declare.

