Abstract
Aims: Renal denervation has emerged as a treatment option for patients with drug-resistant hypertension. This study was designed to assess the safety and effectiveness of the OneShot™ Renal Denervation System.
Methods and results: RAPID is a prospective, multicentre, single-arm study which enrolled 50 patients at 11 clinical sites in Europe and New Zealand. Eligible patients had an office systolic blood pressure (SBP) ≥160 mmHg and were on a stable regimen of ≥3 antihypertensive medications including a diuretic. The primary safety endpoints were acute procedural safety at discharge and chronic procedural safety at six months. The primary effectiveness endpoint was the rate of office SBP reduction ≥10 mmHg at six months compared to baseline. While not a predefined endpoint, change in 24-hour ambulatory BP was evaluated. The mean baseline office SBP and diastolic BP measurements were 181.6±20.8 and 95.5±15.5 mmHg, respectively. Patients were on a mean of 5.1 antihypertensive medications at baseline. The mean office BP decreased by –20/–8 mmHg (p<0.0001/p=0.0002), and –22/–8 mmHg (p<0.0001/p=0.0014), from baseline to six and 12 months, respectively. The 24-hour ABPM was also significantly reduced by –11/–6 mmHg at six months compared to baseline (p=0.0085/p=0.037). There were no serious adverse events (SAE) at discharge related to groin and vascular access complication or renal artery injury or SAE/adverse device effects at six months.
Conclusions: The results of the RAPID study demonstrate safe delivery of RF energy by the OneShot Renal Denervation System for renal sympathetic denervation and sustained efficacy, as evidenced by a significant reduction in office and 24-hour ABPM for six months, which was sustained up to 12 months. ClinicalTrials.gov Identifier: NCT01520506
Abbreviations
ABPM: ambulatory blood pressure measurement
ADE: adverse device effects
BP: blood pressure
CEC: clinical events committee
DBP: diastolic blood pressure
RAAS: renin-angiotensin-aldosterone system
RDN: renal denervation
RF: radiofrequency
RFG: radiofrequency generator
SAE: serious adverse events
SBP: systolic blood pressure
Introduction
Hypertension remains an important global concern and a significant risk factor for complications including myocardial infarction, stroke, retinopathy, heart and renal complications1. In 2000, an estimated one billion people worldwide had hypertension, and this global burden was predicted to increase to 1.56 billion by 20252. An estimated 14%-30% of patients are categorised as having resistant hypertension, meaning that, despite adherence to the prescribed antihypertensive regimen, their blood pressure (BP) remains well above goal3,4. The challenges to effective hypertension management include poor patient compliance and adherence to the prescribed therapeutic regimen, and significant side effects associated with the agents. Consequently, novel approaches to manage resistant hypertension are of the utmost importance.
Renal sympathetic denervation has emerged as a promising minimally invasive treatment option for patients with resistant hypertension5,6. Recent studies have shown that this percutaneous, catheter-based approach is safe and effective7-12. However, the efficacy of the denervation procedure has been brought into question by the latest results from the SYMPLICITY HTN-3 trial13. Results from HTN-3 showed safety and effectiveness of the renal denervation procedure in a Caucasian (non-black) cohort of patients with resistant hypertension. The response of African-Americans enrolled in the HTN-3 study suggested they may have a different pathophysiology regarding their hypertension, already mirrored by different drug regimens, relying less on RAAS blockade when compared to their Caucasian counterparts. In addition, there was an unexplained strong “placebo” effect observed in the African-American sham control group not observed in previous studies13.
The OneShot™ Renal Denervation System (OneShot system; Covidien, Mansfield, MA, USA) is an irrigated RF balloon catheter delivering energy in a circumferential manner to achieve denervation of renal arteries using a single two-minute ablation to each renal artery. Previous studies, including HTN-3, were carried out with first-generation percutaneous catheters which require multiple point-by-point ablations per artery, thus significantly prolonging the procedure time. The OneShot irrigation is designed to mitigate damage to non-target arterial tissue, effectively allowing the energy to penetrate deeper into the tissue at the target treatment area, possibly increasing effectiveness14. Nakagawa et al demonstrated in a canine model that irrigation of the ablation electrode with saline cools the tissue, maintaining a low electrode-tissue interface temperature. In their study, the maximal lesion depth in the animals treated with irrigation was located 4.1 mm from the surface compared to 1.2 mm below the surface in the temperature control group (p<0.01)14. Similarly, Sakakura et al, in a study of perfusion-fixed human renal artery specimens, revealed that the distribution of perirenal nerves varied by region examined. The mean number of nerves in the proximal and middle regions was similar, and significantly more than those in the distal segment (p=0.01). Additionally, the mean nerve distance to arterial lumen in the proximal, middle and distal segments was 3.4, 3.1 and 2.6 mm, respectively15.
The feasibility and safety of ablation with the OneShot system was initially shown in nine patients enrolled in the Renal Hypertension Ablation System (RHAS) trial16,17. RF ablation with the OneShot catheter resulted in a significant reduction in office BP that was sustained up to 12 months. In this first-in-man study, the complication rate was low and angiographic assessments at six months after the procedure did not reveal any vascular abnormalities16.
The aim of this prospective, multicentre study was to evaluate the safety and efficacy of the OneShot Renal Denervation System with irrigation for the treatment of patients with chronic resistant hypertension.
Methods
STUDY DESIGN
The Rapid Renal Sympathetic Denervation for Resistant Hypertension Using the OneShot™ Ablation System (RAPID) study is a prospective, multicentre, non-randomised study to evaluate the safety and effectiveness of the OneShot™ Renal Denervation System (Covidien) for the treatment of chronic resistant hypertension. This study was approved by the ethics committees at all participating centres prior to site activation.
PATIENTS
Eligible patients were 18 to 85 years old, had office SBP ≥160 mmHg despite being treated with ≥3 antihypertensive medications, including a diuretic. Patients were required to be stable on their antihypertensive regimen 14 days prior to enrolment, and medication changes were discouraged up to the six-month follow-up visit. Obligatorily, the renal arteries had to be between 4 and 7 mm in diameter with a 20 mm length landing zone for balloon placement.
Patients were excluded if they had end-stage renal disease, a glomerular filtration rate less than 45 mL/min/1.73 m2, type 1 diabetes mellitus, and bleeding disorders. Patients with myocardial infarction, unstable angina, coronary events or stroke within six months of the treatment were excluded. Also, patients with serious renal abnormalities including severe renal artery stenosis, or evidence of prior renal stenting performed and presence of more than one main renal artery (not meeting the protocol-defined anatomical criteria) were excluded. All patients provided written informed consent.
RENAL IMAGING
Patients underwent renal artery imaging at baseline to satisfy the anatomical requirements and in order to evaluate for post-procedural renal artery stenosis. Acceptable imaging modalities included renal duplex, magnetic resonance imaging, computed tomography angiography (CTA) and renal angiography. At the six-month follow-up visit, imaging was performed to assess the treatment area. The same modality was encouraged for both time points. Additionally, duplex renal artery ultrasound imaging was collected for the first 20 enrolled subjects at the one and 12-month follow-up visits.
FOLLOW-UP
Follow-up assessments occurred at one, three, six and 12 months following the renal denervation procedure. Each follow-up visit included assessments of office BP, heart rate, weight, laboratory tests, review of antihypertensive medications and adverse events. Following the denervation procedure, patients were asked to provide information on pain. Patients reporting pain were asked to rank their pain on a scale from one to 10, with one being the least pain and 10 being the most pain experienced.
STUDY DEVICE
The OneShot™ Renal Denervation System comprises the OneShot™ radiofrequency (RF) balloon catheter and a radiofrequency generator (RFG). The system has been described previously16,17. It consists of an irrigated RF balloon catheter and an RFG with an integrated pump (Figure 1). A monopolar silver electrode is painted on the non-compliant balloon in a helical configuration (Figure 1A), ensuring delivery of RF energy in a spiral pattern without the need to manipulate the catheter. The balloon is delivered to the renal artery over a conventional 0.014” interventional guidewire through a guide catheter, 6 Fr or 7 Fr depending on the size of the balloon. Radiopaque markers identify the balloon ends for positioning under fluoroscopy. Balloon catheters are available in 5, 6 and 7 mm balloon diameter sizes and the balloon length is 20 mm.
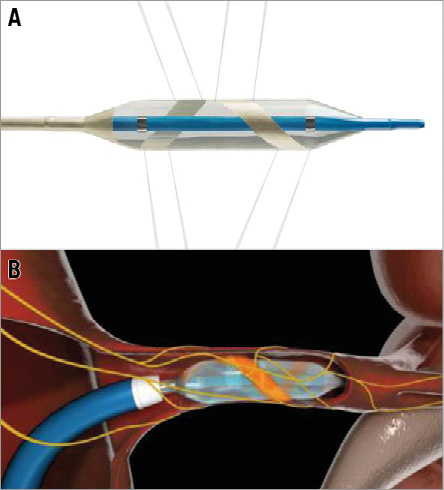
Figure 1. OneShot™ Renal Denervation System. The OneShot™ RF balloon catheter with a spiral RF electrode and irrigation holes. A) Low-pressure irrigation runs continuously (depicted by eight grey lines) during balloon inflation, providing cooling of the non-treated region of the artery during ablation. B) Schematic representation of left renal artery cutaway showing inflated balloon with spiral electrode (orange).
The balloon is inflated to nominal size by a flow of 10 ml/min saline delivered by the pump integrated into the RFG, corresponding to a target pressure of 1 atm. The inflated balloon stabilises electrode contact with the renal arterial wall. During ablation, saline seeps from irrigation holes which are present along the electrode. These are designed to mitigate damage to non-target arterial tissue (Figure 1B). A single, two-minute, 25W RF ablation is delivered to each artery.
ENDPOINTS
The primary safety endpoints of the study were:
1. acute procedural safety, defined as the overall rate of serious adverse events (SAE) and adverse device effects (ADE) at discharge:
a) SAE related to groin and vascular access complications, and
b) SAE related to renal artery injury;
2. chronic procedural safety, defined as the overall rate of SAE and ADE at six months.
The primary efficacy endpoint was procedural effectiveness, defined as the rate of office SBP reduction ≥10 mmHg at six months compared to baseline. Measurements were performed in triplicate with the patient sitting down, and averaged per protocol definition. An independent clinical events committee reviewed and adjudicated all adverse events in the study.
STATISTICAL ANALYSIS
The study is a multicentre, prospective, single-arm clinical study with intra-patient comparisons enrolling 50 patients with chronic resistant hypertension. Descriptive statistics were provided for baseline demographics, procedural characteristics and study outcomes. Changes in BP at follow-up visits from baseline were evaluated using the paired t-test. A p-value of <0.05 was defined as statistically significant. All statistical analysis was performed using Statistical Analysis System (SAS) for Windows version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
BASELINE CHARACTERISTICS
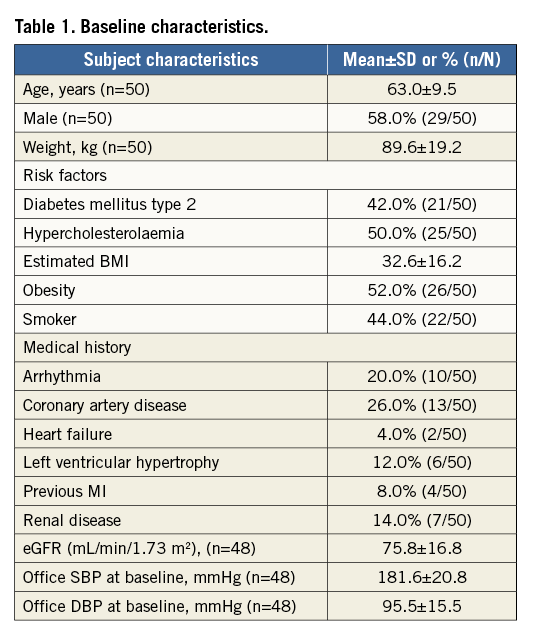
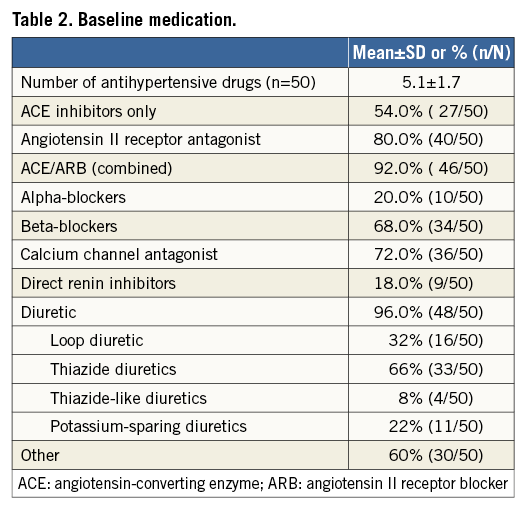
Fifty patients with resistant hypertension were enrolled, treated with the OneShot system and followed up to 12 months (Figure 2). Baseline demographic and clinical characteristics and medication use are presented in Table 1 and Table 2. Patient characteristics were consistent with resistant hypertension, patients were 63.0±9.5 years of age, 58.0% were male, and mean office SBP and diastolic blood pressure (DBP) values were 181.6±20.8 and 95.5±15.5 mmHg, respectively. On average, patients were taking 5.1±1.7 antihypertensive medications, with 96% of patients on a diuretic. Two patients were enrolled despite not taking a diuretic as mandated in the protocol. Additionally, two patients were enrolled and treated who did not meet the inclusion criteria for office SBP ≥160 mmHg. Prior to the three-month follow-up visits it was identified and determined, consistent with the protocol, that these patients would be followed for safety but excluded from the efficacy analysis.
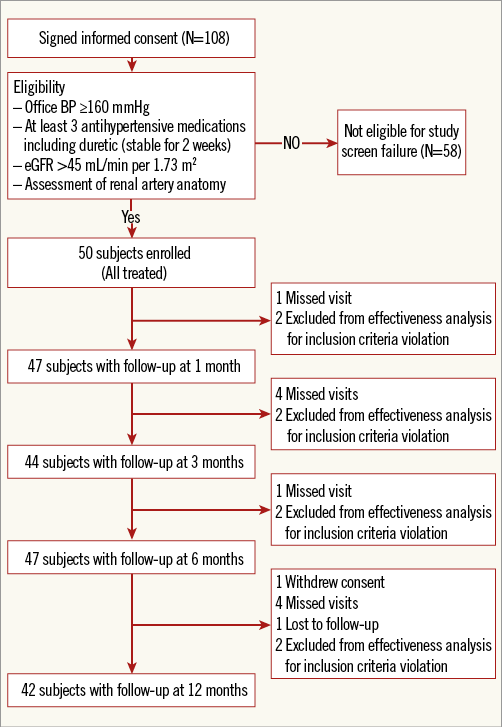
Figure 2. Study flow chart. Represents the number of patients who completed each follow-up visit.
PROCEDURE CHARACTERISTICS
The median procedure time, defined as time from initial arterial access to closure, was 48 minutes (range 32-71 minutes), and the median OneShot system ablation time was 4.0 minutes for the treatment of both arteries. The median fluoroscopic time was 8.0 minutes, and mean contrast volume used was 105.0 mL (range 79.0-150.0 mL). Centres used their locally established protocol for sedation and analgesia, predominantly consisting of fentanyl, midazolam and propofol.
SAFETY ENDPOINTS
All reported adverse events were reviewed by an independent clinical events committee and adjudicated for seriousness and relatedness to the study procedure or device. For acute procedural safety, there were no SAE or ADE at discharge related to groin and vascular access complication or renal artery injury. Two events, flank pain and bradycardia, occurred in two patients during the procedure. Both patients were treated successfully and recovered without further complications. For chronic procedural safety, there were three device- and/or procedure-related SAE reported up to 12 months (Table 3). The three events were infection at access site due to use of a closure device, paraesthesia of the right groin and renal artery stenosis.
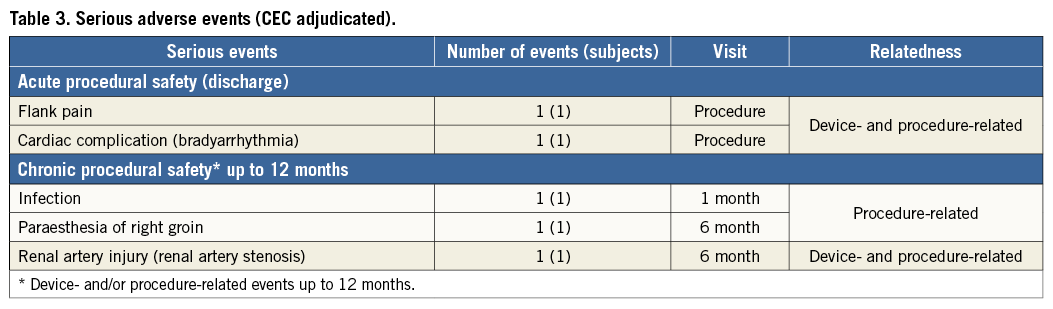
RENAL IMAGING
Renal artery imaging studies were conducted pre-procedure to determine enrolment eligibility and at six months after renal denervation to identify any renal abnormalities. In addition to the standard imaging modality conducted per centre, the first twenty subjects also underwent renal imaging by duplex ultrasonography imaging at one and 12 months post procedure. By this method, no renal abnormalities were observed at baseline which precluded the patients from receiving treatment by denervation.
Imaging studies were available for 43 patients at the six-month follow-up visit including: CTA scans in 17 patients, magnetic resonance imaging in 20 patients and duplex scans in six patients. In one patient, CTA imaging identified a non-haemodynamically significant (<50%) stenosis of the left renal artery which had not been observed at initial treatment. Repeat angiographic imaging with fractional flow reserve and OCT showed that the lesion was within the treatment area, was non-flow-limiting and renal function was not impaired. The patient was asymptomatic at the time of six-month evaluation, and there were no changes in clinical status at the 12-month visit. Twenty patients at one month and 14 patients at 12 months had duplex imaging studies performed, and no additional renal abnormalities were identified.
MEDICATION ADHERENCE
Per study protocol, patients were required to maintain baseline doses of their antihypertensive medications up to the six-month visit. Study sites documented any changes in medication, whether an addition, removal, or dose change to a patient’s antihypertensive regimen. Twenty-two study subjects (44%) had no reported changes in their hypertensive medications during the six-month follow-up period. Of these patients, the responder rate was 64% and the mean change in office BP was similar (–19/–5 mmHg) to that reported for the entire cohort. The patients with a response to the ablation at the six-month follow-up were on an average of 5.1 medications compared to 4.4 in the group that did not respond to ablation. Conversely, over the six-month follow-up period, 28 (56%) patients reported a change in one or more antihypertensive medications at either interim office follow-up visit one, two or three. Of the 28 patients, 11 (39.3%) had a decrease in their antihypertensive medications and 14 (50%) had an increase in their antihypertensive medications at six months.
EFFICACY ENDPOINT - CHANGE IN BLOOD PRESSURE
Office BP reduction was assessed at one, three, six and 12 months (Figure 3). Renal denervation with the OneShot system resulted in a significant reduction in both SBP and DBP. The mean reductions in BP were –17/–7, –17/–7, –20/–8, and –22/–8 mmHg (p<0.0001/p=0.0009, p=0.0002/p=0.0014, p<0.0001/p=0.0002, and p<0.0001/p=0.0014) at one, three six, and 12 months, respectively. At all time points after denervation, SBP and DBP measurements were significantly lower when stratified by age group (≤65 vs. >65 years) and gender, and for patients with or without type 2 diabetes (Figure 4). However, the BP reductions did not differ significantly between groups.
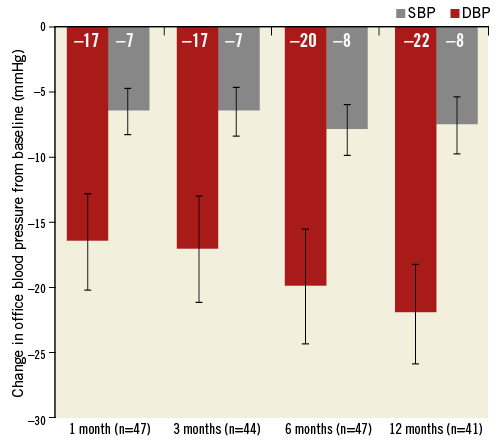
Figure 3. Change in mean office blood pressure at one, three, six and 12 months. Error bars are standard error. The numbers in parenthesis indicate the number of patients with data at each visit (p-values for change in systolic/diastolic BP from baseline to one, three, six and 12 months p<0.0001/p=0.0009, p=0.0002/p=0.0014, p<0.0001/p=0.0002 and p<0.0001/p=0.0014, respectively).
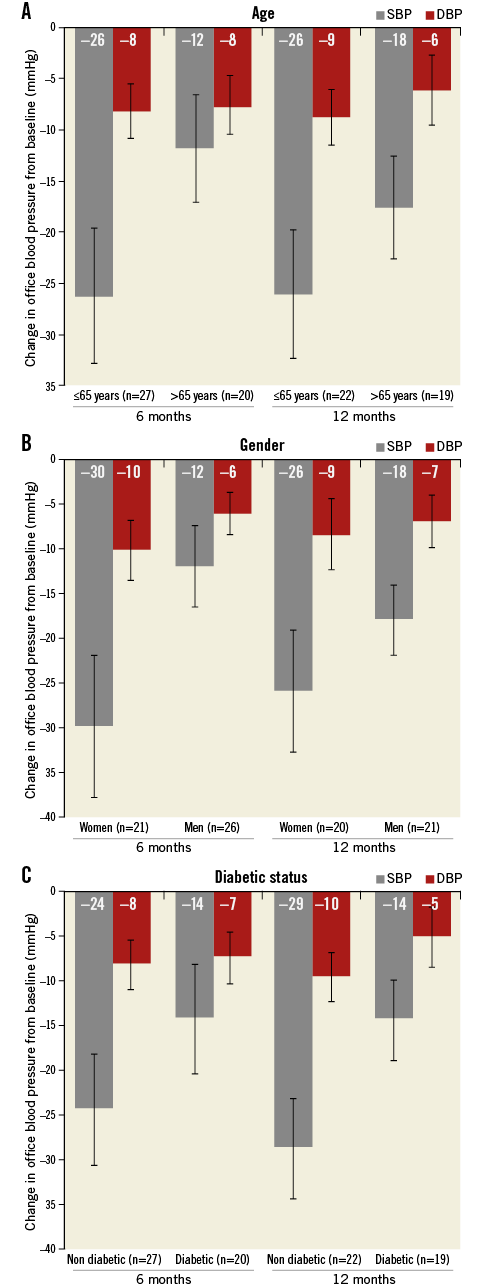
Figure 4. Change in mean office blood pressure by age, gender, and diabetic status. Error bars are standard error. The numbers in parenthesis indicate the number of patients with data at each visit. (Between-group p-values for change in BP at six and 12 months were not significant).
The responder rate, defined as the rate of office SBP reduction ≥10 mmHg at six months compared to baseline was 61.7% (29/47). Over the six-month follow-up period, additional post hoc analysis revealed that 70.2%, 53.2% and 46.8% of patients had BP reductions of at least 5, 15 and 20 mmHg, respectively. Of the non-responders, 11 (61%) patients reported changes of one or more antihypertensive medications.
CHANGE IN AMBULATORY BLOOD PRESSURE MEASUREMENT
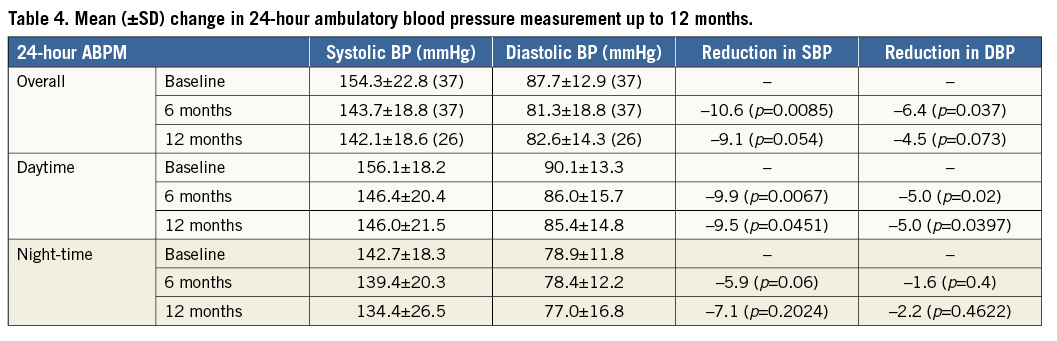
Ambulatory blood pressure measurement was not a predefined endpoint in this study but the data were collected at baseline and at all follow-up visits up to 12 months. Thirty-seven patients at six months and 26 patients at 12 months had evaluable matched ABPM post renal denervation. There was a statistically significant mean change in ABPM of –11/–6 mmHg (p=0.0085/p=0.037) at six months (Figure 5, Table 4). Moreover, there was a significant change in mean systolic and diastolic daytime ABPM, which was absent at night time (Table 4). The change in ABPM was sustained to 12 months (–9/–5 mmHg, p=0.054/p=0.073), but this difference did not attain significance.
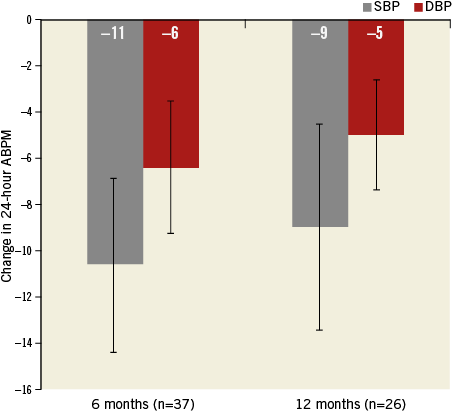
Figure 5. Change in ambulatory blood pressure measurement up to 12 months. Error bars are standard error. The numbers in parenthesis indicate the number of patients with data at each visit. (p-values for change in systolic/diastolic BP from baseline to six [p=0.0085/p=0.037] and 12 [p=0.054/p=0.073] months).
PAIN MANAGEMENT
Pain management was at the discretion of the operator. In general, the procedure was performed with conscious sedation and intravenous narcotics. Twenty-seven (54%) patients reported no pain during the ablation process compared to 23 (46%) patients who reported experiencing pain. In four cases of no pain, the patients were fully sedated. Patients who reported feeling pain were asked to rank their pain on a score from 1 to 10, where one was the least pain and 10 the most pain. The mean pain score reported was 7.9.
Discussion
The RAPID study evaluated the safety and effectiveness of the OneShot system for treating patients with resistant hypertension. Effectiveness was studied by comparing baseline BP values to follow-up values in an intra-individual comparison. This irrigated ablation system was successfully used in the 50 patients treated and resulted in a significant reduction in systolic and diastolic BP at all pre-specified time points up to 12 months. Similarly, ABPM was significantly reduced at six months post denervation. No periprocedural serious access-site complications were reported.
The results show a satisfactory safety profile for the OneShot system. Few adverse events were related to the study device at six months. Eighty-six percent of patients underwent non-invasive renal artery imaging after six months which revealed one incidence of new stenosis. Rigorous review of the event both at six and at 12 months showed that it required no further treatment and renal function was not impaired in the patient.
The OneShot balloon catheter system allows for a single two-minute delivery of RF energy by the spiral electrode, providing a reliable, circumferential, and consistent treatment approach. This differs from the point-by-point ablations utilised by other RF systems such as Symplicity™ (Medtronic, Palo Alto, CA, USA) and EnligHTN (St. Jude Medical, St. Paul, MN, USA). It is difficult to make direct procedure time comparisons between systems. Unlike the RAPID study where total procedure time (defined as time from arterial access to closure) is reported, other studies only report time for RF application. The median OneShot total procedure time was 48 minutes. Likewise, the median OneShot system RF application time was four minutes for bilateral renal artery treatment. In contrast, the Symplicity system requires four or more point ablations, with RF energy applied at each point for two minutes. The median procedure time, defined as time from initiation to completion of RF energy, reported in the SYMPLICITY HTN-1 study was 38 minutes8. Worthley et al reported a similarly defined median procedure time of 34 minutes with the EnligHTN system. Arguably, the shorter procedural time leads to abbreviated painful stimuli without compromising the effects of the therapy.
Another unique feature of the OneShot system is the presence of irrigation. The balloon has eight evenly spaced holes along the electrode. During the ablation procedure, saline seeps out, cooling the surrounding tissue. Irrigation has been shown to minimise fluctuations in temperature at the tissue-electrode interface, to reduce thrombus and char formation, and to prevent damage of the endothelial lining14. Furthermore, irrigation has been suggested to create deeper lesions14. The depth of lesions created by an irrigated ablation system corresponds nicely with the general distribution of nerves reported by Sakakura et al15.
Despite these differences in device, the reductions in BP at six and 12 months observed in the RAPID study confirm earlier results from the RHAS (the first-in-human trial) and are consistent with recently published studies using RF energy8,12,16. A significant reduction in office BP was evident at one month (–17/–6 mmHg) and preserved up to 12 months (–22/–8 mmHg). Though the study was not powered to detect intergroup differences in BP reduction, post hoc analysis revealed no significant changes in BP by age group, gender, or diabetic status. Of interest, there was an observed trend towards an increased magnitude in BP change for younger patients (<65 years), women and non-diabetic patients, which persisted up to 12 months. Due to the small sample sizes for these subgroups, further studies are needed to understand whether these differences could impact on BP and whether octogenarians, men, and diabetics are poor candidates for renal denervation.
Although we show an overall significant drop in office BP for the entire cohort, 38% (n=18/47) of patients were non-responders at six months. This is similar to recent registry reports describing a non-responders rate of around 30% with the Symplicity system18. Of the non-responders, 61% reported changes of one or more antihypertensive medications at six months. A small subset of non-responders (five patients) had directionally positive results, with a mean drop in BP of –5.4 mmHg. This was below the predefined responder threshold of 10 mmHg. The rest showed increases in office BP at six months. It is unclear why the procedure did not show efficacy at six months in these patients. There has been evidence of late response in patients at later time points10,19.
Lack of medication adherence during the six-month follow-up period could have confounded the results of the study. Over half of the patients followed at six months reported changes in their baseline antihypertensive medication regimen. Per protocol, no official testing, i.e., urinalysis, was performed to assess medication levels specifically at follow-up visits. However, patients were instructed to stay on their baseline regimen during the six-month follow-up period. No additional data were captured on adherence up to 12 months.
Renal denervation also led to a substantially significant decrease in 24-hour ABPM (–11/–6 mmHg) at six months. As expected, this effect was less marked than the corresponding change in office BP (–20/–8 mmHg) at six months. Nevertheless, this represents a marked reduction when compared to former drug studies showing ABPM reductions of around 5 mmHg (i.e., valsartan studies)20. An attenuated 24-hour ABPM effect has been observed in other studies8,10,12. The difference in APBM was sustained up to 12 months but did not attain statistical significance.
There remains a need for clarity and definition about which patients are best suited for treatment with renal denervation.
Limitations
Limitations of the study include the lack of a control arm. This single-arm study was designed specifically to assess the safety and effectiveness of the OneShot system as assessed by intra-individual comparisons. Additional studies with a larger sample size and a randomised control arm would be essential to elucidate better the benefits of this therapeutic modality. Finally, adherence to the baseline drug regimen was a requirement of the study that was not closely followed by 56% of the enrolled patients and general practitioners. This could have confounded the results.
Conclusions
In conclusion, results of the RAPID study confirmed that renal denervation with the OneShot system is safe and effective for the treatment of patients with resistant hypertension.
| Impact on daily practice |
Acknowledgements
We wish to acknowledge all investigators and staff at the centres who participated in this study. We thank Prof. Dr Joseph Andre Yves Dens, Ziekenhuis Oost-Limburg, Belgium; Dr Michiel Voskuil, Universitair Medisch Centrum, The Netherlands; and Dr Joost Daemen, Erasmus MC Thoraxcenter for their contributions to the study. The RAPID study was sponsored by Maya Medical, later purchased by Covidien. Special thanks to Karen Krygier for study management, Mei Jiang for statistical support, Jacqueline Mahal for analysis of antihypertensive medication use, and Azah Tabah for technical review of the manuscript.
Funding
Covidien, Mansfield, MA, USA.
Conflict of interest statement
J. Ormiston was a minor shareholder in Covidien who acquired Maya Medical, the company which developed the OneShot system. The other authors have no conflicts of interest to declare.

