Abstract
Background: Ultrasound and radiofrequency renal denervation (RDN) have been shown to safely lower blood pressure (BP) in hypertension.
Aims: The TARGET BP OFF-MED trial investigated the efficacy and safety of alcohol-mediated renal denervation (RDN) in the absence of antihypertensive medications.
Methods: This randomised, blinded, sham-controlled trial was conducted in 25 centres in Europe and the USA. Patients with a 24-hour systolic BP of 135-170 mmHg, an office systolic BP 140-180 mmHg and diastolic BP ≥90 mmHg on 0-2 antihypertensive medications were enrolled. The primary efficacy endpoint was the change in mean 24-hour systolic BP at 8 weeks. Safety endpoints included major adverse events up to 30 days.
Results: A total of 106 patients were randomised; the baseline mean office BP following medication washout was 159.4/100.4±10.9/7.0 mmHg (RDN) and 160.1/98.3±11.0/6.1 mmHg (sham), respectively. At 8 weeks post-procedure, the mean (±standard deviation) 24-hour systolic BP change was -2.9±7.4 mmHg (p=0.009) versus -1.4±8.6 mmHg (p=0.25) in the RDN and sham groups, respectively (mean between-group difference: 1.5 mmHg; p=0.27). There were no differences in safety events between groups. After 12 months of blinded follow-up, with medication escalation, patients achieved similar office systolic BP (RDN: 147.9±18.5 mmHg; sham: 147.8±15.1 mmHg; p=0.68) with a significantly lower medication burden in the RDN group (mean daily defined dose: 1.5±1.5 vs 2.3±1.7; p=0.017).
Conclusions: In this trial, alcohol-mediated RDN was delivered safely but was not associated with significant BP differences between groups. Medication burden was lower in the RDN group up to 12 months.
Introduction
Hypertension (HTN) remains a major cardiovascular risk factor, affecting approximately one-third of adults worldwide1. Lowering systolic and diastolic blood pressure (BP) to recommended targets is associated with a substantial reduction in cardiovascular outcomes including stroke, heart failure, and myocardial infarction2. HTN management is challenging because of non-adherence to prescribed antihypertensive medications and lifestyle interventions, and more recently by the coronavirus disease 2019 (COVID-19) pandemic13456.
The renal sympathetic nerves are involved in the development and maintenance of HTN78. Catheter-based renal denervation (RDN) using radiofrequency or ultrasound energy has been demonstrated to safely lower BP in patients not receiving910 or receiving1112 antihypertensive medications.
The Peregrine System Infusion Catheter (Ablative Solutions, Inc.,) delivers microdoses (0.6 mL per treatment site) of dehydrated alcohol, as a neurolytic agent, locally into the perivascular space of the renal artery to achieve ablation of the afferent and efferent sympathetic nerves13141516. A previous open-label trial using this catheter demonstrated that alcohol-mediated RDN was delivered safely and significantly lowered ambulatory and office BP in patients with severe uncontrolled HTN taking medications13. The TARGET BP program is a series of randomised, sham-controlled, assessor-blinded trials investigating the safety and efficacy of alcohol-mediated RDN for the treatment of uncontrolled HTN in the absence (TARGET BP OFF-MED) or presence (TARGET BP I, pivotal) of antihypertensive medications17. We report the results of the multicentre, blinded, sham-controlled, TARGET BP OFF-MED trial (ClinicalTrials.gov: NCT03503773) at 2 months and up to 12 months of follow-up.
Methods
This randomised, blinded, sham-controlled, trial conducted in 25 trial centres in Europe and the USA was approved by national regulatory authorities and local independent ethics committees (IECs)/institutional review boards (IRBs).
INFORMED CONSENT AND ELIGIBILITY
Patients provided written informed consent and underwent eligibility screening assessments. Patients (18-80 years old) with a mean office systolic blood pressure (SBP) between 140 and 180 mmHg and a mean diastolic blood pressure (DBP) ≥90 mmHg who were taking 0-2 antihypertensive medications were recruited. Patients entered a 4-week run-in period during which they took no antihypertensive medications leading up to randomisation. Before randomisation, patients were required to have a mean 24-hour ambulatory systolic blood pressure (ASBP) of 135-170 mmHg with ≥70% valid readings (determined by ambulatory blood pressure monitoring [ABPM]). Patients with 1 or more accessory renal arteries that were deemed too small for treatment (<4 mm diameter), but supplying >20% of the renal parenchyma, were excluded. A complete list of eligibility criteria is presented in Supplementary Appendix 1.
RANDOMISATION AND PROCEDURE
After confirmation of their anatomical eligibility, patients were randomised in a 1:1 ratio to either the alcohol-mediated RDN or sham control. Randomisation was stratified by trial site and was performed centrally using an interactive web response system. Patients were blinded to treatment status by sensory deprivation and sedation during the procedure. The patients, the sponsor, and the outcome assessors who performed the screening and follow-up assessments, were blinded up to 12 months post-procedure. The interventionalist performing the procedure, and associated personnel, were unblinded but not involved in patient follow-up. Unblinding to treatment assignment and ABPM results took place after the last patients had completed the 12-month follow-up visit. Patient blinding effectiveness was assessed using a treatment perception questionnaire and the James and Bang blinding indices1819 (Supplementary Table 1).
If an anatomically suitable renal artery anatomy was confirmed, patients were randomised to receive RDN using the Peregrine Catheter (RDN group) or diagnostic renal angiography only (sham control group). Significant renal accessory arteries (supplying >20% perfusion of the renal parenchyma) that were 4-7 mm in diameter were also treated, with a maximum of 1 accessory artery treated per side. Each treatment involved administration of 0.6 mL alcohol per treated renal artery with a maximum dose of 2.4 mL alcohol per patient. Total procedure time was defined as the time from femoral artery access to sheath removal.
For those patients randomised to the RDN group, the catheter was inserted via the femoral artery and advanced to the renal artery. Three microneedles were deployed through the media of the vessel, and the alcohol was delivered into the perivascular space surrounding the renal artery. Further details regarding the Peregrine System Infusion Catheter and its use have been previously described13.
FOLLOW-UP
Patient follow-up was conducted at 1, 2, 3 and 6 months and 1 and 2 years post-procedure and included ABPM, office BP, and safety assessments (adjudicated by a clinical events committee [CEC] and reviewed via an independent Data Safety Monitoring Board [DSMB]). At 6 months post-procedure, renal duplex ultrasound, computed tomography angiography (CTA), magnetic resonance angiography (MRA), or renal angiography were performed to assess renal artery patency and the presence of new stenosis. Adherence to the discontinuation of antihypertensive medications per protocol was assessed by tandem high-performance liquid chromatography and mass spectroscopy of urine and plasma by an independent laboratory at baseline and 2 months20. Antihypertensive medication utilisation was assessed by the mean number of antihypertensive medications prescribed, the daily defined dose (DDD), medication index, and the proportion of patients on ≥2 antihypertensive medications. Prescribed antihypertensive medications were summarised according to the sum of the DDD to assess and compare the total drug consumption between groups21. The prescribed dose of each antihypertensive medication was divided by the DDD, which was summed across all prescribed medications. The medication index was defined as a composite index based on the doses of medications and is a proportional measure of prescribed to maximum daily dose, as recommended by the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure22, calculated for each antihypertensive medication.
The primary efficacy endpoint was the change in the mean 24-hour ASBP from baseline to 8 weeks post-procedure as compared between the RDN and the sham control groups. After 8 weeks, antihypertensive medications were titrated to a target office SBP of ≤140 mmHg according to a protocol-defined titration scheme (Supplementary Appendix 2). A full list of study endpoints in provided in Supplementary Appendix 3.
The safety endpoint was the occurrence of major adverse events (MAE) up to 30 days post-procedure. MAE included all-cause death, end-stage renal disease, significant embolic event resulting in end-organ damage or requiring intervention, major vascular complications, major bleeding events, postprocedural renal artery stenosis (>60% diameter stenosis), hypertensive crisis, and symptomatic hypotension requiring medication. Device success was defined as the ability to insert the catheter into the lumen of the renal artery (target vessel), deploy the guide tubes inside the renal artery, deploy the needles through the arterial wall, deliver the intended dose of alcohol, retract the needles and the guide tubes into the catheter, and remove the catheter from the access site without any related complications or events. Procedural success was defined as device success with freedom from periprocedural MAE.
STATISTICAL ANALYSIS
This study was not formally powered for statistical comparisons of efficacy or safety events as this was designed as a proof-of-concept study, the purpose of which was to determine the treatment effect to inform future trial designs. Thus, the sample size was small. The primary efficacy endpoint analysis was conducted on the intention-to-treat (ITT) population and was compared between treatment groups using an analysis of covariance, which was adjusted for the baseline value. The per-protocol (PP) population consisted of patients meeting all eligibility criteria who were not taking antihypertensive medications prior to the primary endpoint collection and did not include RDN group patients with unilateral RDN. The null hypothesis was that there was no difference in the change in 24-hour ASBP between the RDN and sham control groups. The type I error rate for rejecting the null hypothesis is set at a 2-sided alpha level of 0.05. The primary analysis included only available data, and no data imputation was applied. The effect of the COVID-19 pandemic was assessed by exploring results before and after randomisations were paused (11 March 2020). This cutoff date was selected based on coincidence with the implementation of public health measures (e.g., lockdowns) and a pause in study randomisations that followed this date (Supplementary Figure 1).
Results
PATIENT CHARACTERISTICS
Between March 2019 and December 2020, a total of 350 patients were consented, and 106 were randomised (50 and 56 patients in the RDN and sham control groups, respectively) (Figure 1). The majority of patients (81 [76%]; 37 RDN, 44 sham control) were randomised during the COVID-19 pandemic era (the post-COVID group) (Supplementary Figure 1).
Baseline characteristics were similar in both groups (Table 1). Overall, 74% of patients were male with a mean (±standard deviation [SD]) age of 54.1±11.3 years, a mean body mass index of 28.6±4.3 kg/m2, and normal renal function (estimated glomerular filtration rate [eGFR] 85.8±13.4 mL/min/1.73 m2). Before medication washout, the number of patients on 0, 1, or 2 antihypertensive medications was 27 (25.5%), 32 (30.2%), and 47 patients (44.3%), respectively. The baseline mean office SBP/DBP following medication washout was 159.4/100.4±10.9/7.0 mmHg for the RDN group and 160.1/98.3±11.0/6.1 mmHg for the sham control group with a corresponding mean 24-hour SBP/DBP of 147.6/92.2±8.6/7.6 mmHg and 148.8/91.0±9.6/6.8 mmHg for the RDN and sham control groups, respectively (Table 2).
Treatment perception questionnaires, evaluated by the James and Bang indices, indicated successful patient blinding at the time of the procedure and at 8 weeks post-procedure.
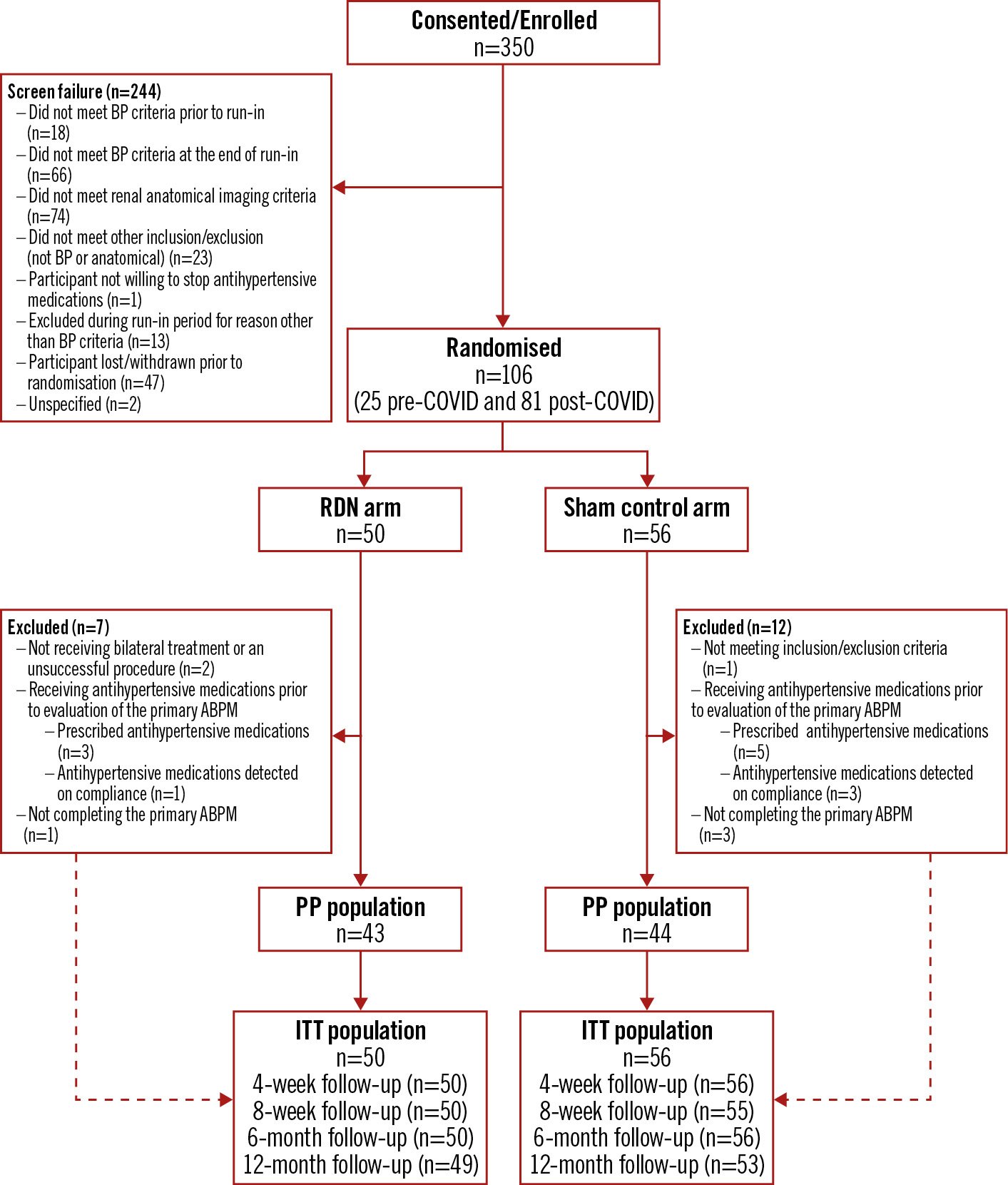
Figure 1. Trial flowchart. The flow of patients through the trial. ABPM: ambulatory blood pressure measurement; BP: blood pressure; COVID: coronavirus disease; ITT: intention-to-treat; PP: per protocol; RDN: renal denervation
Table 1. Baseline patient characteristics.
| RDN (n=50) | Sham control (n=56) | |
|---|---|---|
| Age | 53.8±11.0 | 54.4±11.5 |
| Male | 40 (80.0) | 38 (67.9) |
| Body mass index, kg/m2 | 28.1±4.2 | 28.9±4.4 |
| eGFR, mL/min per 1.73 m2 | 85.8±14.0 | 85.9±13.0 |
| eGFR <60 mL/min per 1.73 m2 | 3 (6.0) | 2 (3.6) |
| Diabetes (all type 2) | 2 (4.0) | 5 (8.9) |
| Smoking (current) | 8 (16.0) | 3 (5.4) |
| Peripheral artery disease | 1 (2.0) | 1 (1.8) |
| Chronic coronary syndrome | 2 (4.0) | 1 (1.8) |
| 24-hour systolic blood pressure, mmHg | 147.6±8.6 | 148.8±9.6 |
| 24-hour diastolic blood pressure, mmHg | 92.2±7.6 | 91.0±6.8 |
| Office systolic blood pressure, mmHg | 159.4±10.9 | 160.1±11.0 |
| Office diastolic blood pressure, mmHg | 100.4±7.0 | 98.3±6.1 |
| Office heart rate, bpm | 76±11 | 77±14 |
| Number of antihypertensive medications at screening | ||
| 0 | 12 (24.0) | 15 (26.8) |
| 1 | 17 (34.0) | 15 (26.8) |
| 2+ | 21 (42.0) | 26 (46.4) |
| Numbers are reported as mean±standard deviation or frequency (percentage). bpm: beats per minute; eGFR: estimated glomerular filtration rate; RDN: renal denervation | ||
Table 2. 24-hour ambulatory and office SBP summary (ITT population).
| Baseline | 8 weeks | 6 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|
| RDN | Sham control | RDN | Sham control | RDN | Sham control | RDN | Sham control | |
| Ambulatory SBP | ||||||||
| Mean±SD, mmHg (n) | 147.6±8.6 (50) |
148.8±9.6 (55) |
144.6±10.1 (48) | 147.0±11.5 (52) | 134.1±11.6 (45) | 135.1±11.7 (48) | 137.6±11.4 (41) | 133.7±11.3 (44) |
| Change from baseline mean±SD, mmHg (n) | –2.9±7.4 (48) |
–1.4±8.6(51) | –13.9±11.6 (45) | –13.4±12.9 (47) | –10.6±11.5 (41) | –15.9±13.1 (43) | ||
| P-value from baseline to 8 weeksa | 0.0089 | 0.25 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Difference between groups (95% CI) | –1.5 (–4.8 to 1.7) | –0.55 (–5.7 to 4.6) | 5.3 (–0.1 to 10.7) | |||||
| P-value for between-group differenceb | 0.2682 | 0.6964 | 0.0775 | |||||
| Office SBP | ||||||||
| Mean±SD, mmHg (n) | 159.4±10.9 (50) | 160.1±11.0 (56) | 155.4±14.3 (50) | 160.6±16.3 (54) | 146.1±16.4 (45) | 145.7±14.3 (51) | 147.9±18.5 (41) | 147.8±15.1 (50) |
| Change from baseline mean±SD, mmHg (n) | –4.0±12.6 (50) |
0.63±13.24 (54) | –12.9±15.6 (45) | –14.7±15.7 (51) | –11.0±15.3 (41) | –13.2±16.6 (50) | ||
| P-value from baseline to 8 weeksa | 0.029 | 0.73 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Difference between groups (95% CI) | –4.6 (–9.7 to 0.4) | 1.8 (–4.5 to 8.2) | 2.2 (–4.5 to 8.9) | |||||
| P-value for between-group differenceb | 0.0605 | 0.724 | 0.6823 | |||||
| Ambulatory DBP | ||||||||
| Mean±SD, mmHg (n) | 92.2±7.6 (50) |
91.0±6.8 (55) |
90.0±7.3 (48) |
90.1±9.7 (52) |
83.0±8.4 (45) |
83.4±9.0 (48) |
85.6±8.7 (41) |
81.0±7.9 (44) |
| Change from baseline mean±SD, mmHg (n) | –2.0±5.1 (48) |
–1.1±6.6 (51) |
–9.3±6.9 (45) |
–8.0±8.5 (47) |
–7.3±7.5 (41) |
–9.8±8.3 (43) |
||
| P-value from baseline to 8 weeksa | 0.0086 | 0.2443 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Difference between groups (95% CI) | –0.9 (–3.3 to 1.4) | –1.3 (–4.5 to 1.9) | 2.5 (–0.9 to 6.0) | |||||
| P-value for between-group differenceb | 0.4734 | 0.5386 | 0.0341 | |||||
| Office DBP | ||||||||
| Mean±SD, mmHg (n) | 100.4±7.0 (50) |
98.3±6.1 (56) |
97.0±9.4 (50) |
97.3±10.9 (54) |
90.4±9.4 (45) |
89.7±10.5 (51) |
91.0±11.0 (41) |
88.5±11.5 (50) |
| Change from baseline mean±SD, mmHg (n) | –3.5±7.6 (50) |
–1.1±8.8 (54) |
–10.0±9.0 (45) |
–8.4±9.5 (51) |
–9.4±9.4 (41) |
–9.6±11.0 (50) |
||
| P-value from baseline to 8 weeksa | 0.0022 | 0.3578 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Difference between groups (95% CI) | –2.3 (–5.6 to 0.9) | –2.5 (–6.1 to 1.2) | –1.6 (–5.4 to 2.1) | |||||
| P-value for between-group differenceb | 0.1843 | 0.3575 | 0.6375 | |||||
| aP-value from t-test of the hypothesis that the change from baseline is different than 0, by visit and trial group. bP-value for comparing RDN and sham control for the difference in the change from baseline from the ANCOVA model adjusted for baseline blood pressure. ANCOVA: analysis of covariance; CI: confidence interval; DBP: diastolic blood pressure; ITT: intention-to-treat; RDN: renal denervation; SBP: systolic blood pressure; SD: standard deviation | ||||||||
EFFICACY RESULTS
At 8 weeks, there was a change from baseline in the 24-hour SBP in the RDN group of â2.9±7.4 mmHg (p=0.009) versus â1.4±8.6 mmHg in the sham group (p=0.25) with a mean difference between groups of â1.5 mmHg (95% confidence interval [CI]: â4.8 to 1.7; p=0.27) (Table 2, Supplementary Figure 2, Central illustration). The change in office SBP from baseline to 8 weeks was â4.0±12.6 mmHg (p=0.03) in the RDN group versus 0.6± 3.2 mmHg (p=0.73) in the sham group with a mean between-group difference of â4.6 mmHg (95% CI: â9.7 to 0.4; p=0.06) (Figure 2, Table 2). A primary endpoint analysis using the per-protocol population was consistent with observations in the ITT population (Supplementary Table 2). The individual 24-hour BP responses are presented in Supplementary Figure 3.
For daytime and night-time ambulatory SBP, the changes from baseline to 8 weeks in the RDN group were â3.2±9.5 mmHg compared with â1.7±9.9 mmHg in the sham group with a mean between group difference of â1.5 mmHg (95% CI: â5.4 to 2.4; p=0.2660) and â3.3±9.4 mmHg in the RDN group versus â0.6±12.2 mmHg in the sham group with a mean between-group difference of â2.8 mmHg (95% CI: â7.1 to 1.6; p=0.1908), respectively.
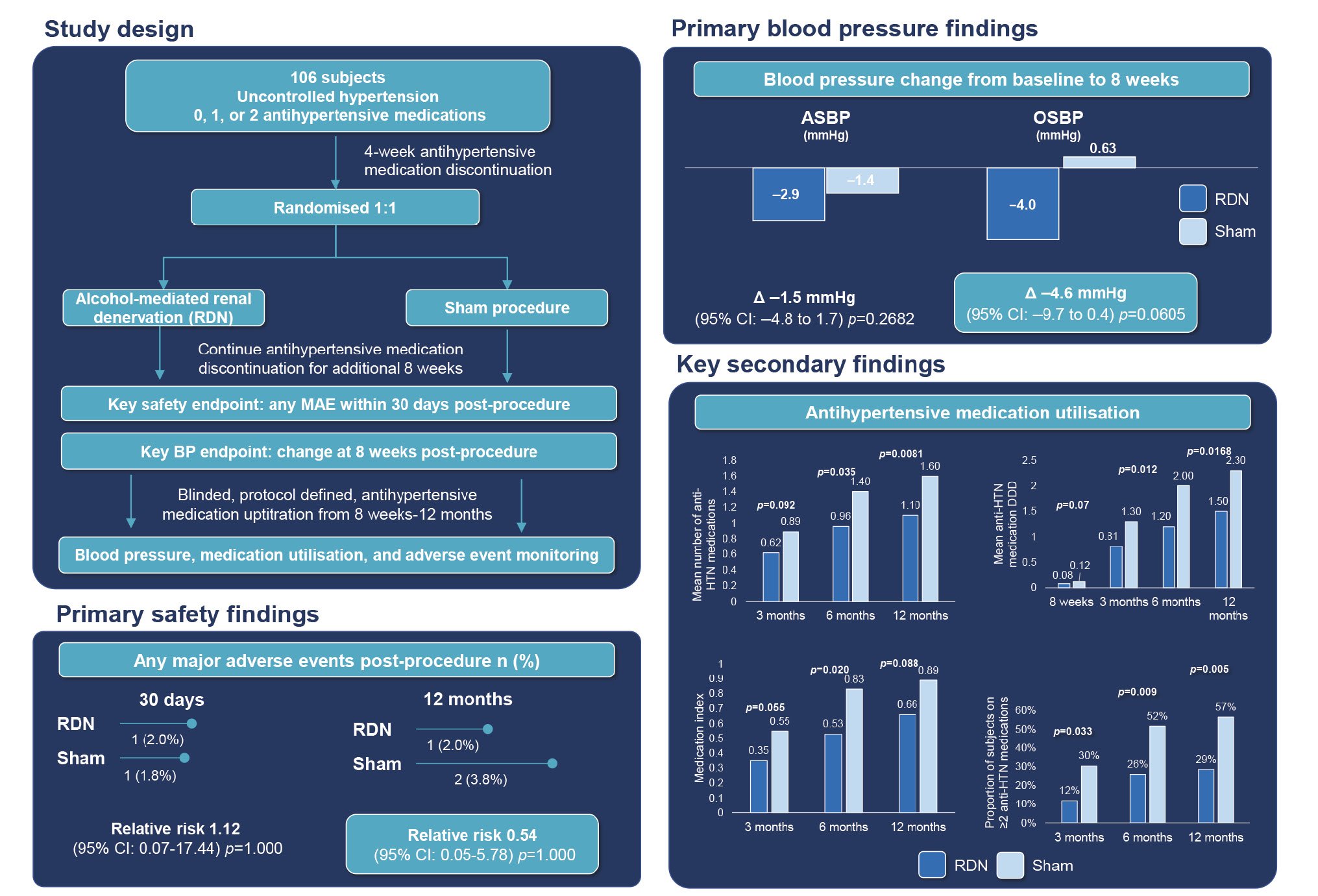
Central illustration. Alcohol-mediated renal denervation effects in the absence of antihypertensive medications. Fifty subjects were randomised to the alcohol-mediated renal denervation arm and 56 subjects were randomised to the sham control. Up to 12 months, there were no differences in safety events between groups. At 8 weeks post-procedure, there were no significant BP differences between groups despite a non-significant trend for a greater office BP reduction in the RDN group. After 12 months of blinded follow-up, the medication burden was lower in the RDN group. ASBP: ambulatory systolic blood pressure; BP: blood pressure; CI: confidence interval; DDD: defined daily dose; HTN: hypertension; MAE: major adverse event; OSBP: office systolic blood pressure; RDN: renal denervation
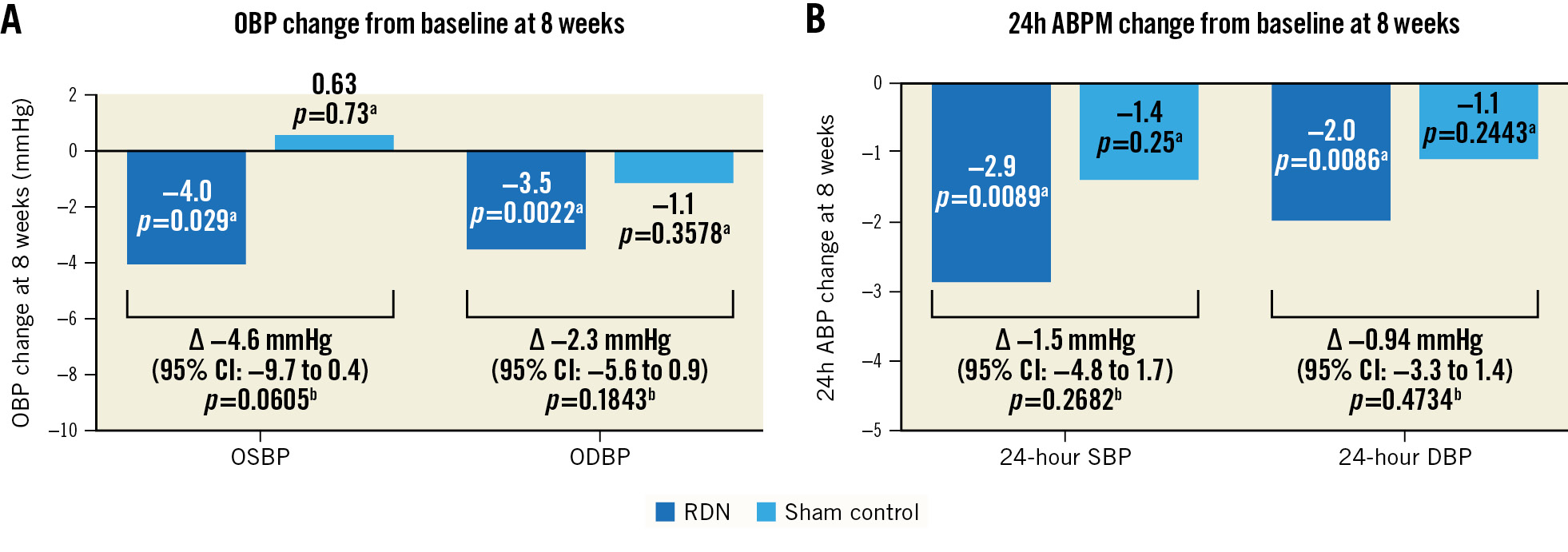
Figure 2. Blood pressure change from baseline at 8 weeks. A) Office and (B) ambulatory BP changes from baseline at 8 weeks post-procedure for the RDN group (blue) and sham control group (light blue). aP-value from t-test of the hypothesis that the change from baseline is different than 0, by trial group. bP-value for comparing RDN and sham control for the difference in the change from baseline from the ANCOVA model adjusted for baseline blood pressure. ABPM: ambulatory blood pressure measurement; ANCOVA: analysis of covariance; CI: confidence interval; DBP: diastolic blood pressure; OBP: office blood pressure; ODBP: office diastolic blood pressure; OSBP: office systolic blood pressure; RDN: renal denervation; SBP: systolic blood pressure
ANTIHYPERTENSIVE MEDICATIONS
Three patients in the RDN group and 5 patients in the sham group were prescribed antihypertensive medications for safety reasons, per the discretion of the treating investigator, before the 8-week 24-hour BP measurement. After 8 weeks, the most widely used antihypertensive medications in the RDN group were calcium channel blockers in 16 (32.0%), 23 (46.0%), and 23 (46.0%) of participants at 3, 6, and 12 months, respectively. This drug class was also the most frequently used of the antihypertensive medications in the sham group at 3 and 6 months, respectively (19 [33.9%] and 24 [42.9%] participants). At 12 months, sham patients used angiotensin II receptor blockers most frequently (26 [46.4%] participants) (Supplementary Table 3).
In addition, antihypertensive drug metabolites were detected in the urine or plasma of 1 RDN and 3 sham group patients. Sensitivity analyses exploring multiple imputation techniques for these data points did not materially alter the conclusions regarding the BP changes (Supplementary Table 4).
Following primary endpoint collection at 8 weeks, antihypertensive medication was uptitrated to achieve a target office SBP ≤140 mmHg while the patient and treating physician were blinded to treatment group assignment up to 12 months post-procedure. Antihypertensive medication utilisation, measured by the mean number of antihypertensive medications prescribed, the DDD, medication index, and the proportion of patients on ≥2 antihypertensive medications, increased from 8 weeks to 12 months in both groups. However, antihypertensive medication use was lower in the RDN group at 3, 6, and 12 months post-procedure (Figure 3). The corresponding BP outcomes are reported in Table 2.
Patients randomised to RDN or sham before the start of the COVID-19 pandemic (pre-COVID group n=13; 11 March 2020) had larger 24-hour SBP decreases at 8 weeks post-procedure than patients randomised during the COVID-19 pandemic (post-COVID group) (RDN: â7.0±7.0 mmHg pre-COVID vs â1.5±7.2 mmHg post-COVID; p=0.02; sham: â5.1±5.8 mmHg pre-COVID vs â0.5±9.0 mmHg post-COVID) (Supplementary Table 5). However, notable BP differences between the groups were not observed during the pre- and post-COVID-19 pandemic periods. Further, baseline SBP appeared to be more variable in the post-COVID group for both the RDN and sham groups (RDN: 24-hour SBP SD: 9.2 mmHg and office SBP SD: 11.5 mmHg; sham: 24-hour SBP SD: 9.9 mmHg and office SBP SD: 11.1 mmHg) than in the pre-COVID group (RDN: 24-hour SBP SD: 6.6 mmHg and office SBP SD: 9.1 mmHg; sham: 24-hour SBP: 8.8 mmHg and office SBP SD: 7.6 mmHg) (Supplementary Table 6).
Further post hoc analysis showed treatment of all renal accessory arteries (n=5) was associated with a larger decrease in 24-hour SBP compared with subjects with untreated renal accessory arteries (n=8) (change from baseline: â6.6 mmHg versus â0.7 mmHg; p=0.0127) (Supplementary Table 7, Supplementary Table 8).
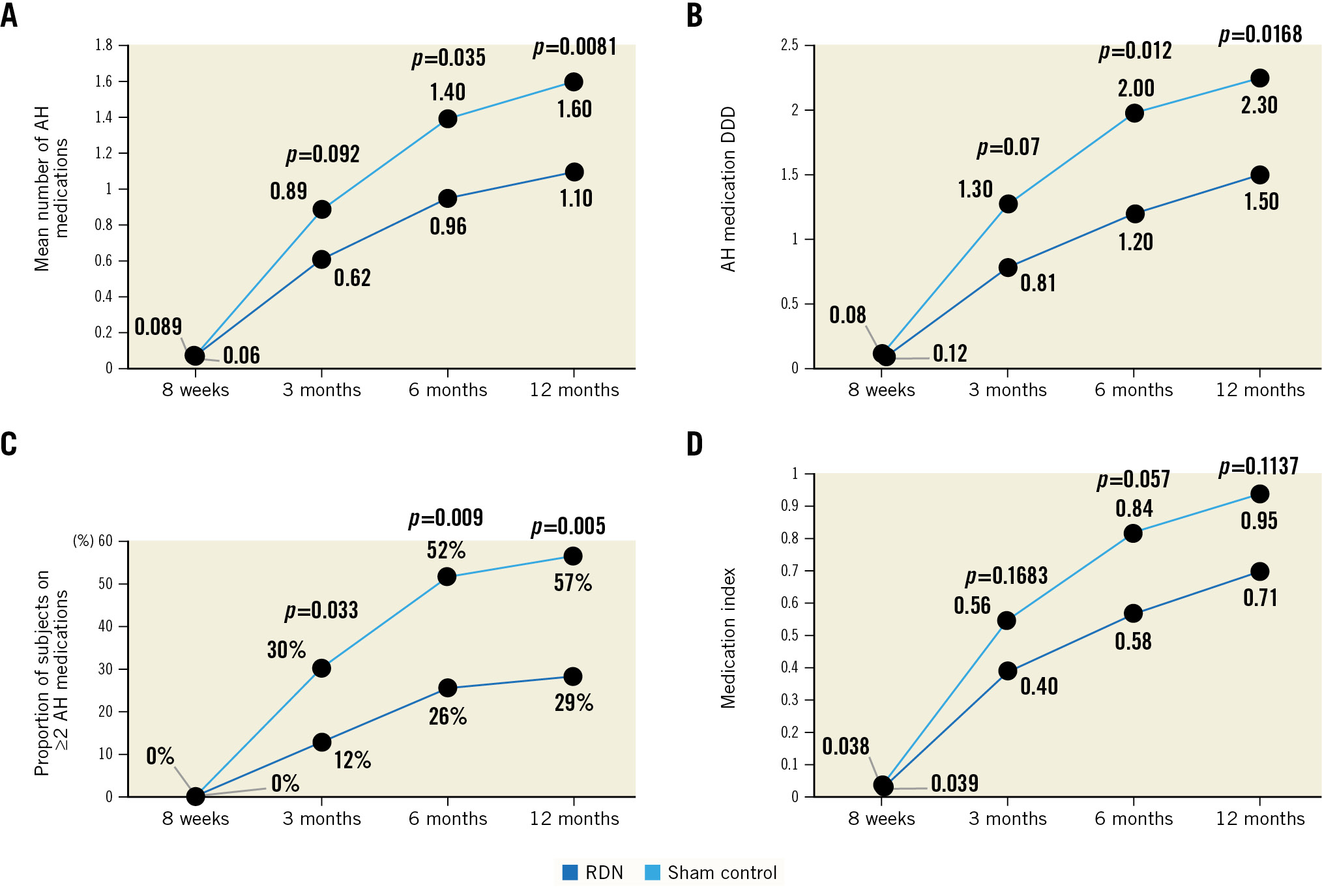
Figure 3. Antihypertensive medication utilisation. Up to 12 months, the RDN group (blue) were on fewer antihypertensive medications than the sham control group (light blue) as measured by (A) the mean number of antihypertensive medications, (B) defined daily dose (DDD), (C) the proportion on ≥2 antihypertensive medications, and (D) antihypertensive medication index. P-value for comparing the RDN group to the sham control group from the t-test for continuous variables and from the chi-square or Fisher’s exact test, as appropriate, for categorical variables. AH: antihypertensive; RDN: renal denervation
SAFETY RESULTS
Forty-eight patients (96.0%) were successfully treated with bilateral, alcohol-mediated RDN using the Peregrine System Infusion Catheter. The mean±SD (range) procedure time was 62.3±24.0 (18-115) minutes with the mean±SD (range) total volume of contrast used 100.0±55.5 (28-300) mL. In 2 patients, challenging anatomies, due to vessel angulation/tortuosity, permitted only unilateral RDN. The incidence of MAE, up to 30 days post-procedure, was similar between groups (RDN: 2.0%, sham: 1.8%). Up to 30 days post-procedure, 1 RDN patient experienced a hypertensive crisis, and 1 sham control patient experienced a vascular complication (the patient developed a small subcutaneous haematoma; aneurysma spurium was subsequently diagnosed). No evidence of renal artery stenosis was identified at 6 months post-procedure via any of the imaging modalities.
eGFR remained stable in the RDN group but decreased in the sham group up to 12 months post-procedure (Supplementary Table 9).
Discussion
The TARGET BP OFF-MED trial investigated the safety and efficacy of alcohol-mediated RDN in hypertensive patients without antihypertensive medications. Alcohol-mediated RDN safety observations were consistent with prior experience with the Peregrine catheter1423 and other RDN modalities924. At 8 weeks post-procedure, the 24-hour ambulatory BP was not statistically significantly different between groups. During blinded follow-up at 3, 6 and 12 months, the use of antihypertensive medication was found to be lower in the RDN group when compared to the sham control group despite similar office BP measurements.
The BP reductions observed in the RDN group were less than those observed in the prior open-label, alcohol-mediated RDN studies with the Peregrine System in patients taking antihypertensive medications1423. After 12 months of blinded follow-up, with medication escalation, office systolic BP values were similar between groups despite a significantly lower medication burden in the RDN group. The mean office systolic BP did not reach guideline-recommended target levels <140 mmHg in either group. However, the present study was a proof-of-concept trial, not formally powered to assess alcohol-mediated RDN in a different, off-medication, study design including a washout period for antihypertensive medications. Based upon prior studies, we anticipated a clinically meaningful change of 5 mmHg between groups2526. Although unlikely (based upon other clinical trial data), one cannot exclude that alcohol-mediated RDN had no effect on BP in patients not taking concomitant antihypertensive medication in this trial cohort. It is important to note that, unlike prior alcohol-mediated RDN studies, the majority of patients in the present trial were recruited during the COVID-19 pandemic. Population-based studies in hypertensive patients during the COVID-19 pandemic have reported increases in SBP as high as 5.6 mmHg456. Other randomised controlled cardiovascular clinical trials have reported similar dichotomous outcomes when subgrouping primary endpoint results by pre- or post-COVID-19 pandemic2728. Similarly, in this trial, larger and clinically meaningful BP changes were observed in patients that were enrolled prior to the start of the COVID-19 pandemic. Results also suggest that 24-hour ABPM may be sensitive to COVID-19 stressors and public health measures which may have affected lifestyle (e.g., sleep deprivation, activity, diet, etc.) and social living. The individual impact is difficult to measure or control in a clinical trial setting. It is possible that this confounding effect was not evenly distributed between patients and treatment groups.
The completeness of renal artery treatment may have also played a role in the smaller than anticipated BP decrease in the RDN group. The treatment of accessory arteries has previously been shown to be related to the magnitude of BP reduction29, and this is consistent with the present trial’s results. This reiterates the importance of complete renal artery treatment on BP reduction, in particular treating accessory arteries, which has been shown to contribute to the sympathetic innervation of the renal parenchyma. For future studies, a Peregrine System Infusion Catheter that facilitates treatment of smaller renal arteries (3-4 mm) is now available and may improve the ability to achieve more complete RDN.
Although the mean office SBP was similar between treatment groups at 3, 6, and 12 months, there were fewer antihypertensive medications used in the RDN group than in the sham control group. Importantly, the reduced antihypertensive medication utilisation in the RDN group occurred while the patients and the treating physicians remained blinded to treatment status. The reduced medication burden observed in the RDN group, relative to the sham control group, may be due to better BP control associated with RDN and a reduced need for medications. This observation suggests an RDN treatment effect and potential benefit for the patient up to 12 months post-procedure30313233.
The pivotal, randomised, powered, sham-controlled TARGET BP I trial (ClinicalTrials: NCT02910414) for patients taking antihypertensive medications is currently ongoing and will further assess the efficacy of alcohol-mediated RDN in the management of HTN.
Limitations
This trial was designed as a hypothesis-generating safety and efficacy trial and, hence, not formally powered for the primary efficacy endpoint. The sample size, in particular for subgroup analyses, was small. Larger, appropriately powered, trials are necessary to conclusively determine the BP-lowering effect of alcohol-mediated RDN in hypertensive patients. Alcohol-mediated RDN with the Peregrine System Infusion Catheter has no intraprocedural operator feedback confirming complete ablation of renal sympathetic nerves. This is currently a limitation for all modalities of RDN. Finally, the COVID-19 pandemic may have introduced additional confounding factors, which, at present, cannot be objectively quantified.
Conclusions
The results from this randomised, sham-controlled, assessor-blinded trial investigating the safety and efficacy of alcohol-mediated RDN for the treatment of uncontrolled HTN in the absence of antihypertensive medications demonstrated that alcohol-mediated RDN was safely delivered; however, there was not sufficient evidence to show a BP difference between groups. The antihypertensive medication burden was lower in the RDN group up to 12 months post-procedure. Studies of larger powered trials, not confounded by the COVID-19 pandemic, are underway to further assess the efficacy of alcohol-mediated RDN in the management of HTN.
Impact on daily practice
Catheter-based RDN using radiofrequency or ultrasound energy has been demonstrated to safely lower BP. Previous open-label trials have demonstrated that alcohol-mediated RDN is safe and has significantly lowered ambulatory and office BP in patients with severe uncontrolled HTN. Despite the results of this current trial not providing sufficient evidence to show a BP difference between groups, the results did demonstrate that alcohol-mediated RDN was safely delivered, and the medication burden was lower in the RDN group up to 12 months post-procedure.
Acknowledgements
Michael Cuchiara and Debbie Brix Reynolds provided editorial support, including copyediting and the creation of figures and tables under the direction of the authors. A list of all participating sites and investigators can be found in Supplementary Appendix 4.
Funding
Ablative Solutions, Inc. was responsible for the funding of this trial.
Conflict of interest statement
A. Pathak received scientific support, support for attending meetings, and speaker honoraria from Ablative Solutions and Medtronic. U.M. Rudolph received speaker honoraria from Novartis, Berlin Chemie, and Bayer; and support from Ablative Solutions to attend educational events. M. Saxena has received institutional grants from Ablative Solutions, ReCor Medical, and Vascular Dynamics; and consulting fees from Esperion and Vifor Pharma. T. Zeller has received study fees to the institution for enrolled patients. R.E. Schmieder has received grants, consulting fees, and honoraria from Ablative Solutions, Medtronic, and ReCor Medical. H. Sievert has received study honoraria to the institution, travel expenses, consulting fees (limited to reimbursement for clinical trials) from 4tech Cardio, Abbott, Ablative Solutions, Adona Medical, Akura Medical, Ancora Heart, Append Medical, Axon, Bavaria Medizin Technologie GmbH, Bioventrix, Boston Scientific, Cardiac Dimensions, Cardiac Success, Cardimed, Cardionovum, CeloNova, Contego, Coramaze, Croivalve, CSL Behring LLC, CVRx, Dinova, Edwards Lifesciences, Endobar, Endologix, Endomatic, Esperion Therapeutics, Inc., Hangzhou Nuomao Medtech, Holistick Medical, Intershunt, Intervene, K2, Laminar, Lifetech, Magenta, Maquet Getinge Group, Metavention, Mitralix, Mokita, Neurotronic, NXT Biomedical, Occlutech, ReCor, Renal Guard, Shifamed, Terumo, Trisol, Vascular Dynamics, Vectorious Medtech, Venus, Venock, and Vivasure. M. Halbech has received consulting fees from Bayer; payment or honoraria from Abbott, AstraZeneca, Bayer, BMS/Pfizer, CTI, CVRx, Daiichi Sankyo, Gilead, Medtronic, MSD, Sanofi-Aventis, Vifor, and ViiV; and support for attending meetings and travel from Amgen, BMS/Pfizer, Boehringer Ingelheim Fonds, CVRx, Orion Pharma, and Servier. H. Parise received consulting fees and institutional support from Ablative Solutions. T.A. Fischell possesses a licence of patents and potential royalties with stock options in Ablative Solutions. M.A. Weber has provided consulting and research services to Ablative Solutions, Medtronic, ReCor, Johnson & Johnson, The George Institute, Bayer/Regeneron, and Omron. D.E. Kandzari has received consulting honoraria and travel support for research activities from Ablative Solutions and Medtronic. F. Mahfoud is supported by Deutsche Gesellschaft für Kardiologie (DGK), Deutsche Forschungsgemeinschaft (SFB TRR219), and Deutsche Herzstiftung. He has received scientific support from Ablative Solutions, Medtronic and ReCor Medical; and speaker honoraria/consulting fees from Ablative Solutions, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Inari, Medtronic, Merck, ReCor Medical, Servier, and Terumo. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

