
Abstract
Aims: The aim of this study was to assess the one-year safety and efficacy of the transcatheter ARTO system in the treatment of functional mitral regurgitation (FMR).
Methods and results: MAVERIC is a multicentre, prospective, non-randomised pre-commercial study. Eligible patients were on guideline-recommended therapy for NYHA Class II-IV systolic heart failure and had an FMR grade ≥2+. The ARTO system was implanted in forty-five (100%) patients. The primary safety composite endpoint (death, stroke, myocardial infarction, device-related surgery, cardiac tamponade, renal failure) at 30 days and one year was 4.4% (95% CI: 1.5-16.6) and 17.8% (95% CI: 9.3-32.4), respectively. Periprocedural complications occurred in seven patients (15.5% [95% CI: 6.5-29.5]), and five patients (11.1% [95% CI: 4.9-24.0]) died during one-year follow-up. Paired results for 36 patients demonstrated that 24 (66.7%) had grade 3+/4+ mitral regurgitation at baseline; however, only five (13.9%) and three (8.3%) patients remained at grade 3+/4+ 30 days and one year post procedure (p<0.0001). Echocardiographic parameters such as anteroposterior annulus diameter decreased from 41.4 mm (baseline) to 36.0 and 35.3 mm at 30 days and one year, respectively (p<0.0001). Twenty-five patients (69.4%) had baseline NYHA Class III/IV symptoms decreasing significantly to nine (25.0%) at 30 days and eight (22.2%) at one year post procedure (p<0.0001).
Conclusions: The ARTO transcatheter mitral valve repair system is both safe and effective in decreasing FMR up to one year post procedure.
Introduction
Functional mitral regurgitation (FMR) is often seen in heart failure (HF) patients with left ventricular (LV) dysfunction and mitral annular adverse remodelling resulting from either ischaemic or non-ischaemic dilated left heart disease1. There is a strong correlation between the severity of FMR and HF hospitalisations and an association with increased all-cause mortality2,3,4. Although pharmacological and/or cardiac synchronisation therapy is beneficial in some patients with FMR, others deteriorate, with surgical mitral valve annuloplasty remaining their only option5,6. Due to comorbidities, these patients are at increased operative risk and are not offered isolated surgical correction unless performed with other cardiac surgeries7,8. While alternatives such as the MitraClip® system (Abbott Vascular, Santa Clara, CA, USA) are available, it is unknown whether it is an appropriate treatment for the majority of HF patients9. Consequently, there remains an unmet need for alternative transcatheter technologies in the treatment of FMR. Initial results of the MitrAl ValvE RepaIr Clinical Trial (MAVERIC trial), using the ARTO™ (MVRx Inc., San Mateo, CA, USA) system, have been reported previously10,11. The device decreases FMR via a suture that connects interatrial-septal and great cardiac vein (GCV) anchors (Supplementary Figure 1). The tension on this suture reduces the anteroposterior (AP) diameter of the mitral annulus, thereby improving mitral leaflet coaptation11. Previous reports have shown reductions in FMR grade, decreased LV volumes, and improved functional class. The positive outcomes achieved for the first 11 patients have allowed the study to be expanded to include a further 34 patients. We report the one-year data of this expanded cohort of 45 patients.
Methods
TRIAL DESIGN AND PATIENTS
MAVERIC is an ongoing, global, prospective, multicentre, non-blinded, non-randomised safety and efficacy study of patients treated for FMR. The primary safety outcome is the major adverse event (MAE) rate 30 days post procedure. MAEs are defined as death, stroke, MI, cardiac tamponade, device-related cardiac surgery and renal failure. Performance endpoints are technical success defined per the Mitral Valve Academic Research Consortium (MVARC)12, mitral regurgitation grade and change from baseline to 30 days as evaluated by two-dimensional (2D) transthoracic echocardiogram (TTE). An independent core laboratory (Cardiovascular European Research Centre [CERC], Massy, France) analysed all echocardiographic data and graded FMR as per the American Society of Echocardiography (ASE) guidelines12. All clinical events were adjudicated by an independent clinical events committee (CEC). Patients are followed at discharge, 30 days, six months, and yearly for three years.
The trial was designed in accordance with the Declaration of Helsinki and registered with ClinicalTrials.gov (Trial ID: NCT02302872). Details of patient eligibility and enrolment have been reported previously and are presented in Supplementary Figure 2,10,11. Additional details are provided in Supplementary Appendix 1 and Supplementary Appendix 2.
STATISTICAL ANALYSIS
This study included a sample size of 45 patients and was powered for two objectives: first, to provide an estimate of the rate of MAEs at 30 days; second, to have >90% power to demonstrate that the upper limit of a two-sided confidence interval for the proportion of patients with an MR grade of 2+ or higher at 30 days is not higher than 50% when assuming that the true proportion of patients with a 30-day MR grade of 2+ or lower is 75%.
Analysis of safety outcomes was performed on an intention-to-treat basis (including all patients who underwent the procedure) using the Kaplan-Meier method, censoring at the time of the outcome, death, or mitral valve replacement.
Analysis of efficacy outcomes excluded patients with an MR grade of 1+ at the echocardiography core laboratory (Supplementary Figure 2), although findings were not sensitive to this choice (data not shown). Comparisons of echocardiographic and functional measurements during follow-up used paired t-tests for continuous and approximately normally distributed variables and used sign-rank tests for ordinal or heavily skewed variables. Analyses were restricted to the set of patients with complete information at all time points. Sensitivity analyses included all patients and used last observation carried forward to impute data in patients who died or did not have measurements.
Statistical tests were two-sided with p-values <0.05 considered statistically significant. Stata 15.1 (StataCorp LLC, College Station, TX, USA) and R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analyses.
Results
The ARTO system was implanted in 45 patients (60% males, 69.8±12.3 years). Baseline characteristics are shown in Table 1 and Supplementary Table 1.
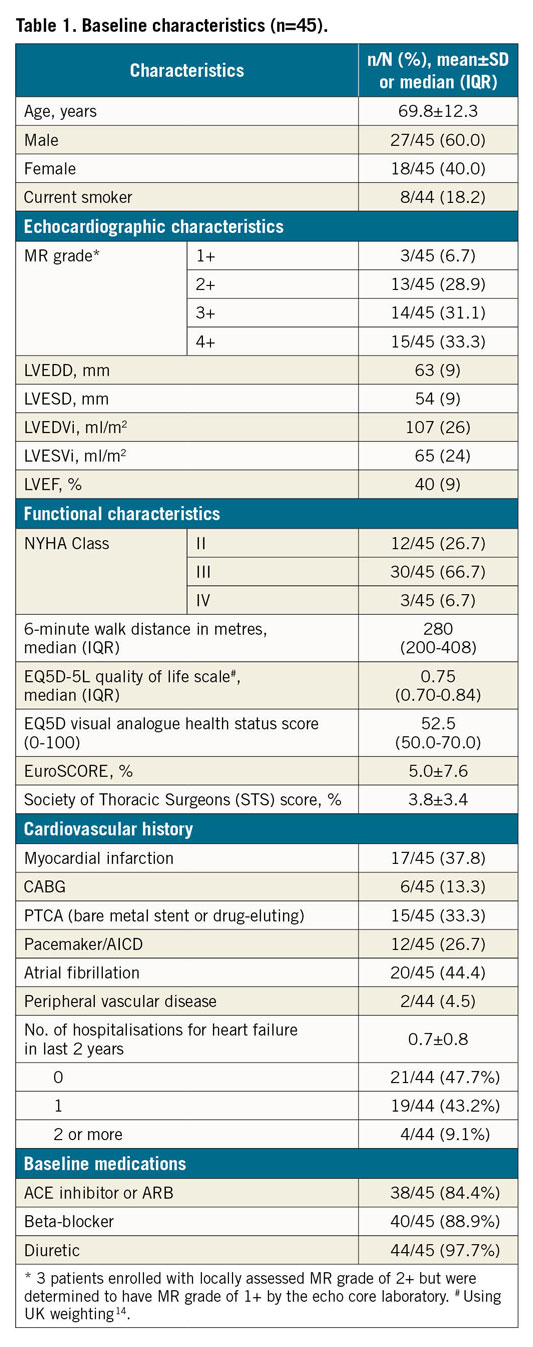
PROCEDURAL CHARACTERISTICS AND OUTCOMES
Median length of the ARTO procedure was 88 minutes (interquartile range [IQR]: 65-110 minutes) and median discharge rate was 2 days (IQR: 1-5 days) (Table 2). Seven patients (15.5%) had procedural complications – 2 minor bleeds, 2 major bleeds and 3 minor vascular access-site complications. One of the major bleeds was due to central line insertion and the other was due to a delayed pseudoaneurysm at the access site resulting in a drop in Hb level of more than 3 g/dL necessitating a transfusion and rendering it a major bleed per MVARC guidelines13. Thus, none of the complications was device-related, and technical success per MVARC12 definition was 100%.
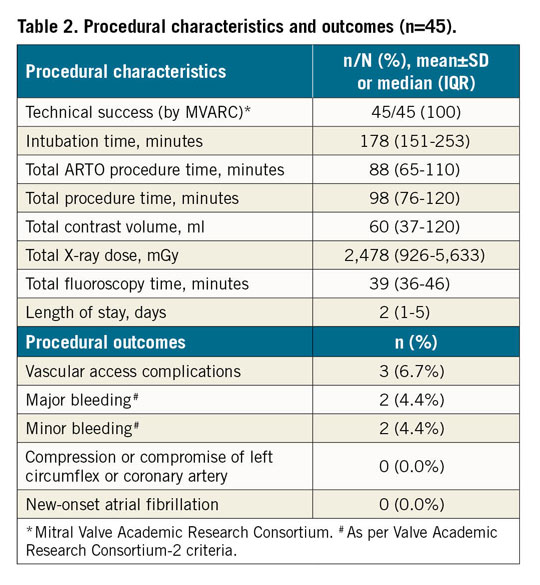
SAFETY OUTCOMES
Thirty-day, six-month and one-year safety outcomes are shown in Table 3 and Figure 1. By day 30, two MAEs (4.4%) were reported. One was a pericardial effusion requiring surgical drainage resulting in complete recovery. In another patient the CT scan was performed two days prior to the ARTO procedure and together resulted in acute kidney injury (AKI) followed by full recovery. This patient died 305 days post procedure from HF deterioration.
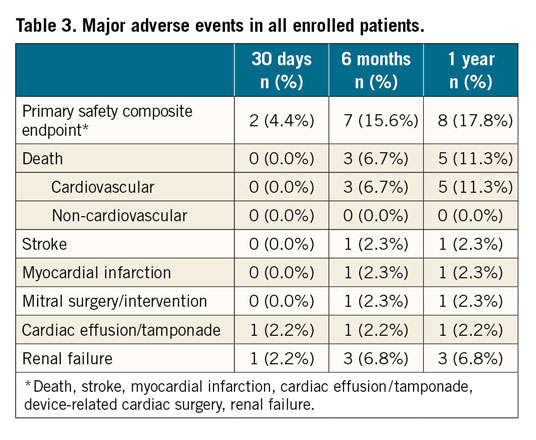
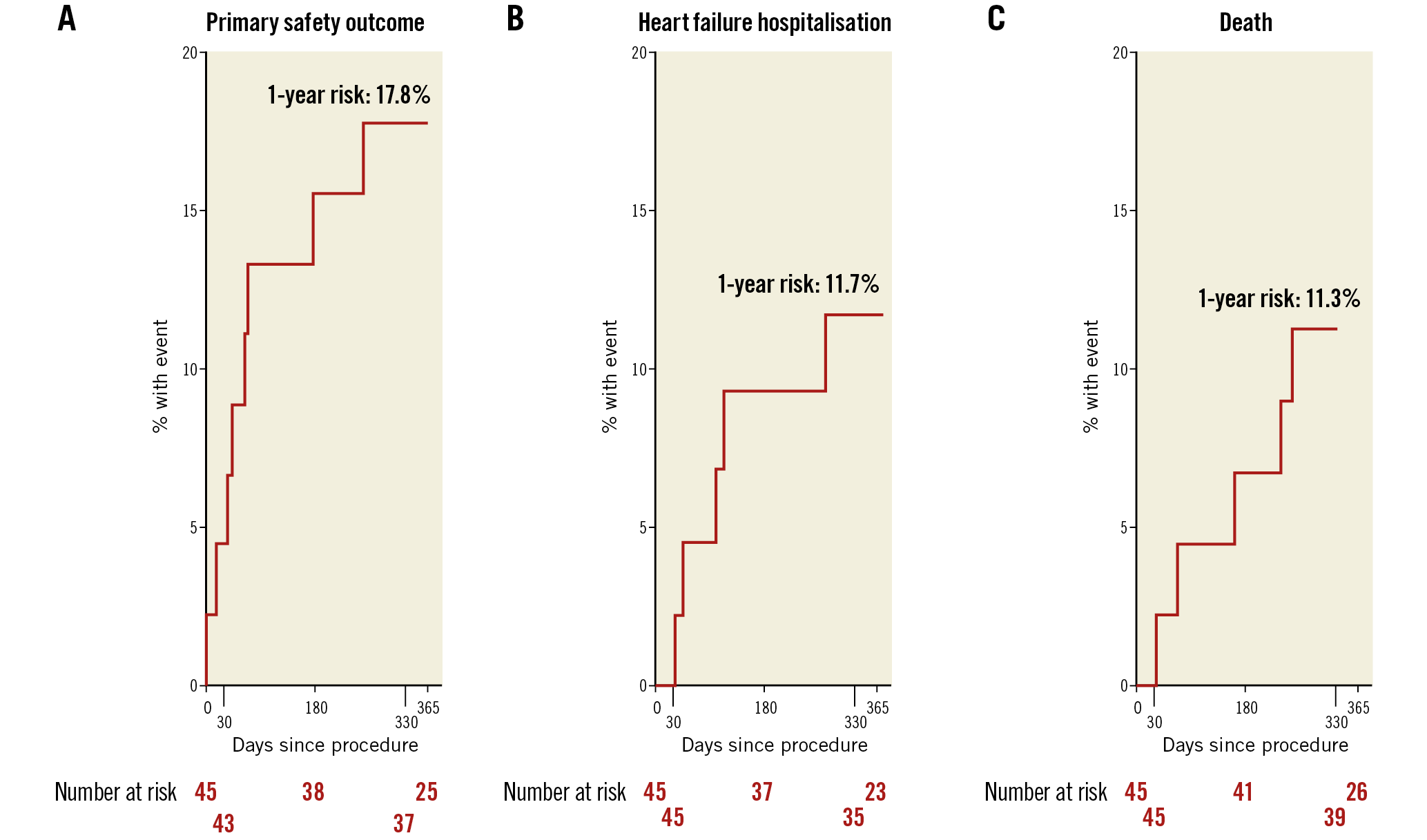
Figure 1. Kaplan-Meier time-to-event curves for the primary safety outcome (A), heart failure hospitalisation (B) and all-cause death (C). The primary safety outcome was defined as death, stroke, myocardial infarction, cardiac effusion/tamponade, device-related cardiac surgery, renal failure.
Seven MAEs from 30 days to six months included three deaths (all adjudicated as cardiovascular unrelated to the device or procedure), one each for stroke, MI, device-related reoperation and renal failure. The causes of the above deaths as per autopsy were either complications arising from ischaemic heart disease or sequelae of HF.
Within one year, MAEs occurred in eight patients (17.8% [95% CI: 9.3-32.4]) including five deaths (11.1% [95% CI: 4.9-24.0]).
EFFICACY OUTCOMES
REDUCTION IN MR AS ASSESSED BY ECHOCARDIOGRAPHIC PARAMETERS
MR reduced significantly at 30 days compared to baseline (p<0.0001) (Table 4, Figure 2), with no evidence of any change in MR grade between 30 days and one year (p=0.30). In paired analyses of 36 patients, MR grade 3+/4+ was present in 24 (66.7%) at baseline, 5 (13.9%) at one month and 3 (8.3%) at one year (p<0.0001). Significant improvements were noted in other echocardiographic indices at one month compared to baseline (Table 4, Figure 2), including a 13% decrease in AP diameter (41.4 vs 36.0 mm, p<0.0001), 47% decrease in regurgitant volume (44.8 vs 23.2 ml, p=0.0001), 48% decrease in effective regurgitant orifice area (EROA; 26.9 vs 13.9 mm2, p=0.0001) and 33% improvement in vena contracta (6.0 vs 4.0 mm, p<0.0001), all of which were sustained at one year post procedure.
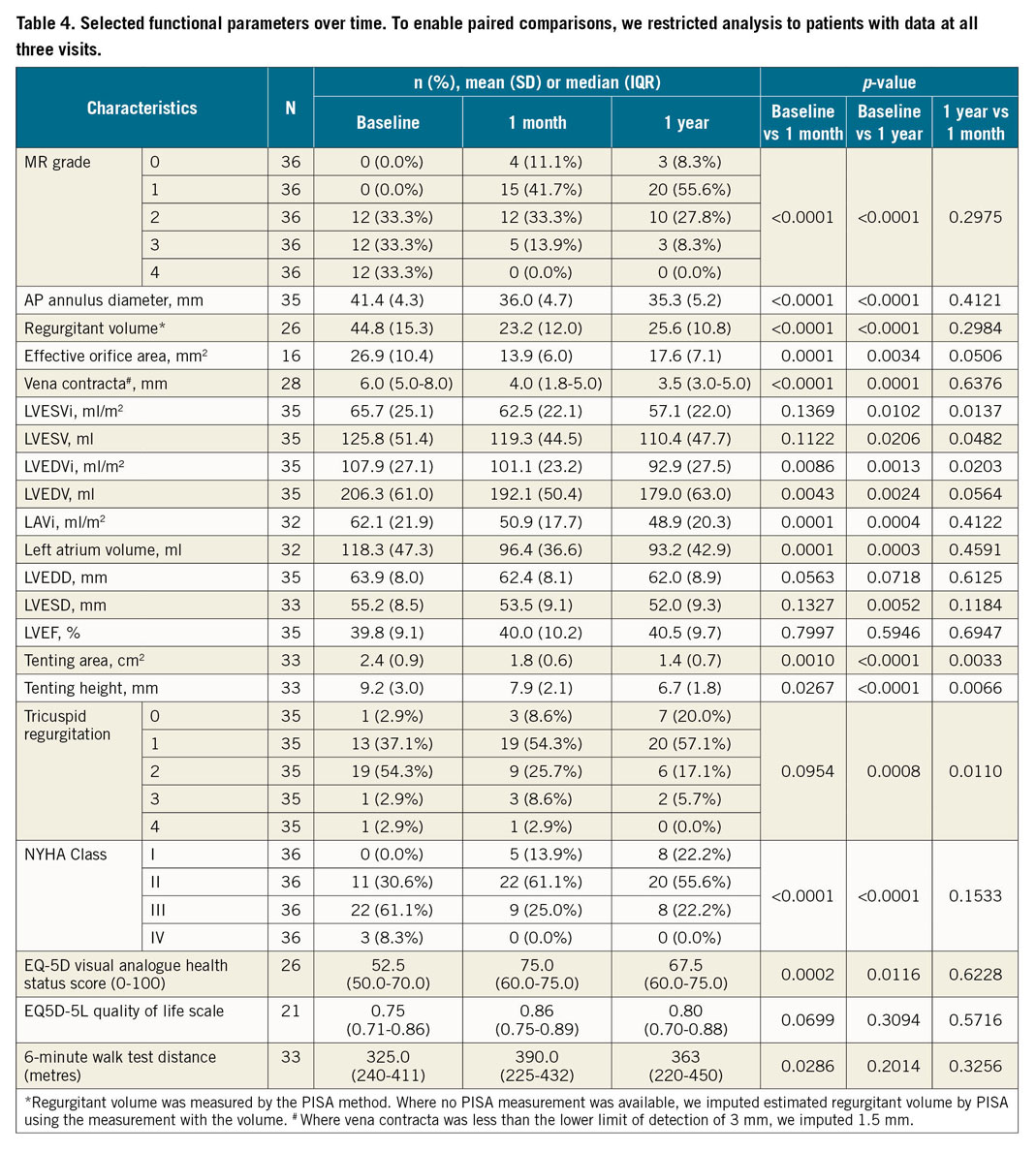
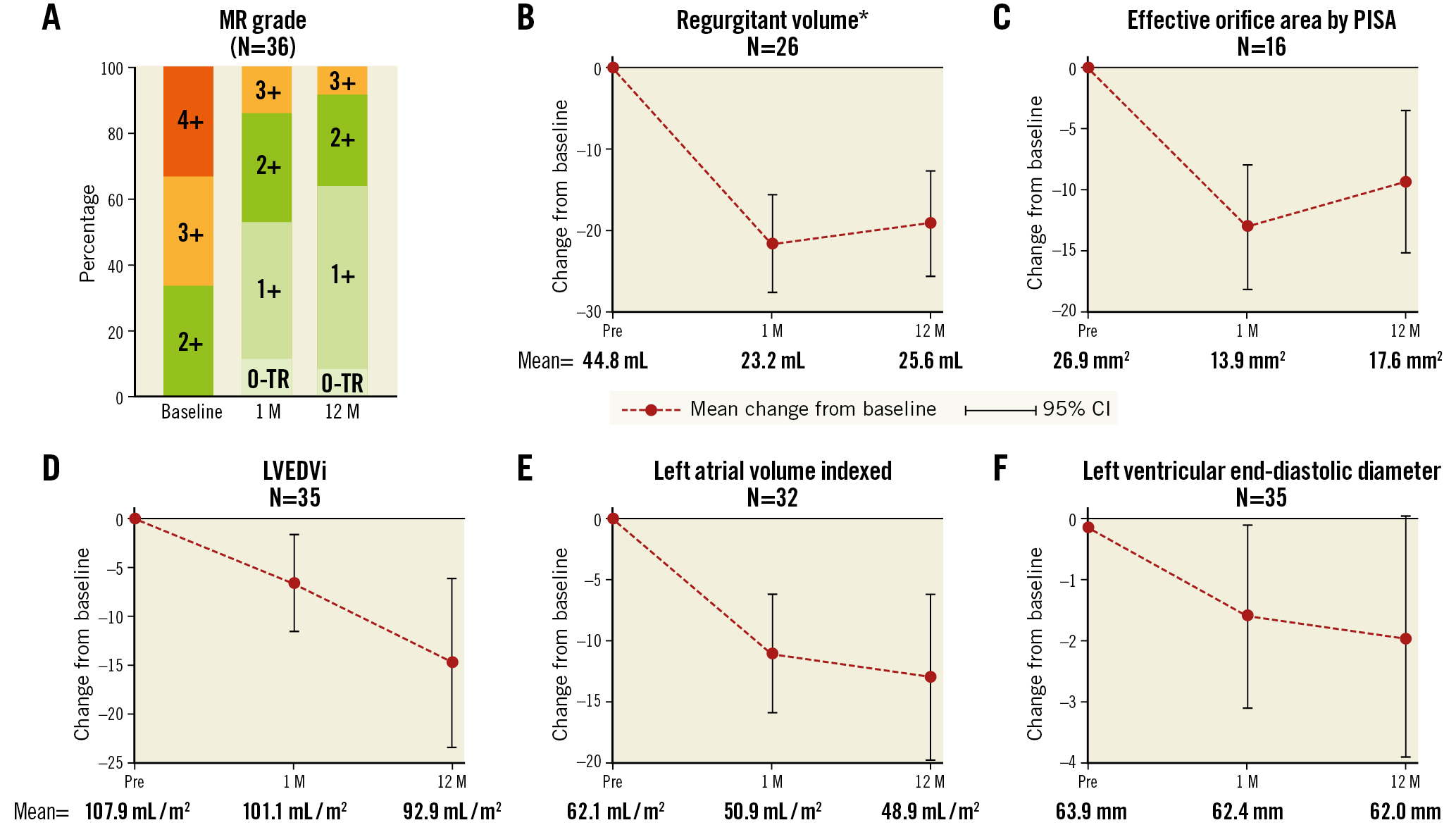
Figure 2. Selected change in echocardiographic parameters between baseline visit and 30-day or one-year visits. MR grade (A) at baseline, one month (1 M) and 12 months (12 M). Echocardiographic parameters: regurgitant volume (B), effective orifice area by PISA (C), LV end-diastolic volume (LVEDVi) (D), left atrial volume indexed (E) and LV end-diastolic diameter (F). P-values are from paired comparisons between time points. *Regurgitant volume was measured by the PISA method. Where no PISA measurement was available, regurgitant volume was estimated by using the measurement with the volume method.
Improvements in cardiac function one year post procedure were demonstrated by a 13% decrease in LV end-systolic volume (LVESVi; 65.7 vs 57.1 ml/m2, p=0.0102), 14% decrease in LV end-diastolic volume (LVEDVi; 107.9 vs 92.9 ml/m2, p=0.0013) and 21% reduction in left atrial volumes (62.1 vs 48.9 ml/m², p=0.0004), all indexed to body surface area.
FUNCTIONAL IMPROVEMENTS POST PROCEDURE
NYHA class improved whereby 8 patients (22.2%) were classified as NYHA Class III/IV at one year, compared to 9 (25.0%) at 30 days and 25 patients (69.4%) at baseline (p-value for improvements in NYHA <0.0001 for both classes). Additionally, there were no significant changes in medication use (Supplementary Table 2), and there were only five HF-related hospitalisations (11.7% [95% CI: 5.1-25.9]). Significant improvements at 30 days compared to baseline were reported in the EQ-5D self-assessed health status (visual analogue scale from 0-100, median 75% at 30 days vs median 52.5% at baseline, p-value=0.0002)14. Paired analyses for six-minute walk tests (6MWT) in 43 patients showed ~16.67% improvement in distance covered at 30-day follow-up compared to baseline (p=0.0286); no further improvements were observed from 30 days to one year (p=0.20) (Supplementary Figure 3). Findings were similar in sensitivity analyses (Supplementary Table 3).
Discussion
The MAVERIC study, designed to assess the safety and efficacy of the ARTO transcatheter mitral valve repair system, showed that the device was a safe therapeutic option for patients15. One-year follow-up data showed that patients implanted with the ARTO system had significant echocardiographic and functional improvements.
Imaging parameters such as EROA ≥20 mm2, left ventricular end-diastolic diameter (LVEDD) >65 mm, regurgitant volume >30 ml and others have been associated with worse outcomes in FMR patients2,16. Patients with data available at all time points showed 35% and 37% improvements in EROA and regurgitant volumes, respectively, at one year. A reduction in FMR has a positive correlation with reverse remodelling and improved survival17,18. Importantly, MAVERIC imaging assessments revealed significant improvements in cardiac volumes (decrease ≥13% in LVESVi/LVEDVi and decrease ≥20% in atrial volumes), suggestive of early reverse LV remodelling. LVESV reductions >10% have been shown to result in significant decreases in mortality and hospitalisations in advanced HF patients19. Functional improvements were also evident with NYHA class that improved significantly at 30 days and remained significant at one year. Furthermore, at one month there were significant improvements in 6MWT and EQ-5D visual analogue health status scores. This is particularly meaningful when considering that the positive 6MWT outcome reported in the COAPT trial was achieved mainly by deterioration in the control arm9. A technical success rate of 100% indicates a safe procedure and a device success rate of 71.4% is noteworthy for a first-generation device, suggesting that the therapy is comparable to other established percutaneous mitral repair therapies20,21.
The ARTO system has several beneficial features: (1) a short procedure performed entirely at the atrial level decreasing ventricular arrhythmias and preserving haemodynamic stability; (2) preprocedural computed tomography (CT) coupled with a coronary angiogram that helps to determine the anatomy of the coronary sinus and circumflex coronary artery, avoiding coronary artery compression; (3) flexibility to adjust/remove the device prior to lock-and-release of the system as required, and (4) individually tailored decrease in AP diameter with immediate MR reduction.
There is a relatively small learning curve for the procedure that led to minor procedural and device modifications. For example, pericardial effusion was reduced by the replacement of the angled wire with a J-tipped wire11. Dislocation of the GCV T-Bar into the left atrium (LA) detected early in the trial in two asymptomatic patients was either corrected surgically or left untouched, at the investigator’s discretion. An atraumatic tip separation noted at follow-up imaging required no intervention since the patient was asymptomatic and MR reduction of 4+ to 1+ was maintained.
The current landscape of transcatheter repair devices for FMR consists of leaflet approaches such as the MitraClip, and indirect/direct annuloplasty approaches. The clinical experience with the MitraClip procedure and FMR has been well reported and has been shown to demonstrate acute MR reduction, improved functional class and low rehospitalisation, but with ~20% FMR >2+ recurrence at 12 months in patients with challenging anatomies22. The latest data from the STS/TVT registry of FMR patients reported 31.2% one-year mortality, 32.6% HF rehospitalisation and death or HF hospitalisation of 49%23. The MITRA-FR trial using the MitraClip device for severe secondary MR failed to show any significant difference between patients who underwent percutaneous mitral valve repair in addition to receiving medical therapy and those who received medical therapy alone24. This was attributed mainly to significant underlying cardiomyopathy, incomplete correction of MR and the severity of the disease in the trial population. Conversely, the COAPT trial demonstrated a lower rate of hospitalisation, lower mortality, better prognosis and better functional capacity in subjects who received the device in addition to guideline-directed medical therapy compared to those receiving only guideline-directed medical therapy9. This further emphasises the need for novel technologies, especially for those patients who are ineligible for a MitraClip device. The recent one-year outcomes of a cohort of sixty patients using the Cardioband system (Edwards Lifesciences, Irvine, CA, USA) indicated an 87% one-year survival with 66% having freedom from HF and 78% freedom from a secondary intervention24. The outcomes from the randomised REDUCE FMR trial using the Carillon Mitral Contour System® (Cardiac Dimensions Inc., Kirkland, WA, USA) show one-year mortality of 14%, and HF hospitalisations of 11% with 9% having device removal for coronary artery compromise25. Although transcatheter mitral valve replacement is being refined in early clinical trials, further investigations are necessary to establish the role of transcatheter repair versus replacement, since the FMR cohort is at increased risk of morbidity and mortality by virtue of underlying LV dysfunction and comorbidities.
Limitations
A small sample size with no control group is always a limitation in studies such as MAVERIC investigating new technologies. The inclusion criteria required subjects to be ineligible for surgery, implying that patients were at higher risk of mortality, morbidity and worse outcomes. That said, no mortality in this study was associated with the ARTO system or procedure. Although MR grade improved significantly and was assessed by the core lab for all patients, individual parameters were not available for all patients – a well-recognised challenge in evaluating FMR patients25. The first eleven patients in the study were not evaluated by EQ-5D at baseline (protocol amended), hampering our ability to show significant improvements in this parameter.
Conclusions
This study shows that the ARTO system has some outstanding features and results, as demonstrated by short procedure times, 100% technical success as per MVARC criteria, small contrast volumes, 86% survival rates at one year, improved MR grades and a 12% hospitalisation rate. NYHA class improved significantly at 30 days and one year. In summary, these data demonstrate that the ARTO system is safe and effective in reducing MR significantly and improving functional status in patients with symptomatic FMR up to one year.
|
Impact on daily practice Mitral valve surgery is a proven therapeutic option for FMR; however, operative risks remain high in patients with comorbidities. The ARTO transcatheter mitral valve repair system is a less invasive method for FMR treatment that appears to be safe and effective, especially in patients ineligible for other CE-marked devices. |
Funding
MVRx, Inc., San Mateo, CA, USA.
Conflict of interest statement
A. Erglis has received research grants from MVRx, Inc. S. Redwood is a proctor for MVRx, Inc. D. Hildick-Smith is a consultant to MVRx, Inc. All other authors have reported no relationships relevant to the contents of this paper.
Supplementary data
To read the full content of this article, please download the PDF.

