Background
Patients with significant symptomatic functional mitral regurgitation (FMR) typically present in the advanced stages of chronic heart failure and with severe comorbidities and advanced age. In contrast to primary MR, the benefits of mitral valve (MV) surgery are uncertain in such patients, and recurrence of FMR after surgical treatment is reported to be up to 50% two years after surgery. In daily routine, only a small number of FMR patients are referred for surgical valve treatment and there is still an unmet need for low-risk treatment options in this vulnerable patient group.
In this context, the Mitralign (Mitralign Inc., Tewksbury, MA, USA) system offers several potential advantages: it is an annuloplasty device for dedicated use in FMR patients, addressing MV annular dilatation, and transseptal puncture is not needed during the procedure.
Patients planned for transcatheter mitral valve repair with the Mitralign procedure need to undergo a thorough clinical evaluation and detailed echocardiographic assessment, since they typically present at high surgical risk for surgical valve treatment, as evaluated by existing risk scores (STS, EuroSCORE). However, the value of these scoring tools is somewhat limited, since they do not take into account factors such as frailty, severe right ventricular dysfunction or “hostile chest”. Clinical evaluation should assess all other comorbidities (e.g., malignancy, etc.) and their impact on the patient’s life expectancy.
Pre-procedural assessment of patient eligibility
Patients eligible for Mitralign treatment should present with symptomatic chronic FMR grade ≥2 and LV ejection fraction ≥25%, as determined by echocardiography, and be on stable guideline-directed heart failure medication and/or device therapy (Table 1).
The presence and significance of possible coexisting coronary artery disease need to be documented and coronary angiography is mandatory. Transthoracic and transoesophageal echocardiography (TTE/TEE) are the key instruments for the assessment of LV dimensions. A severely dilated or small ventricular cavum might cause steering difficulties and prohibit safe placement of the Mitralign Bident catheter on the ventricular side of the mitral annulus and thus safe annular puncture.
TEE guidelines recommend assessment with standardised views of the mitral valve (4-chamber, 3-chamber, commissural, 3D) from a midoesophageal and/or transgastric position. However, imaging protocols need to be adapted to the individual patient’s heart.
Reconstruction of the 3D volumes allows further anatomical measurements. The ventricular perspective can be important in assessing subvalvular structures.
3D TEE usually produces images with spatial resolution that is superior to that of 3D TTE. For Mitralign patient selection, 3D TEE is most useful for anatomical assessment, quantification, and intraprocedural guidance.
Contrast-enhanced computed tomography (CT) is of further help to determine LV dimensions and to detect possible mitral annular calcifications as they might complicate annular puncture. Of note, mitral annular calcification is a common degenerative process associated with advancing age and other comorbidities and is present in approximately 6% of the general population.
Due to dynamic changes in the configuration of the mitral apparatus and LV throughout the heart cycle, CT data acquisition should preferably image the entire cardiac cycle using retrospectively electrocardiography-gated or prospectively electrocardiography-triggered data acquisition.
Procedural echo guidance
During the procedure, 2D and 3D TEE views are necessary for successful guiding and optimal results. “Surgical” or “reverse surgical” views allow en face visualisation of the mitral valve and implanted pledgets (Figure 1).
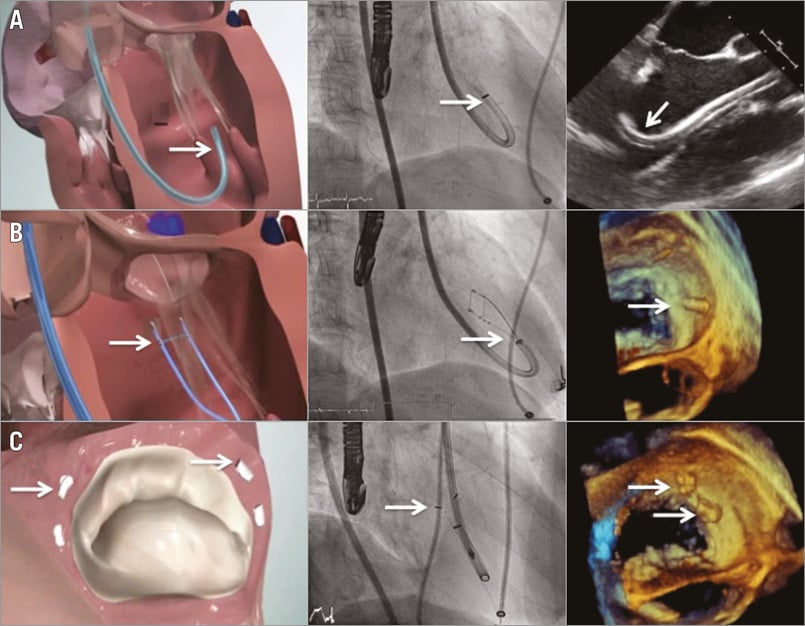
Figure 1. Steps of the Mitralign procedure. A) The guide catheter (white arrows) is placed at the ventricular side of the mitral annulus. B) The pledgets are deployed through the Bident catheter (white arrows) using radiofrequency energy. C) The pledgets (white arrows) are implanted in the mitral annulus and cinched to achieve a reduction in mitral annular anterior-posterior diameter.
Multiplane images from intercommissural (50-70°) and long-axis (120-160°) views are employed for correct and facilitated placement of the 14 Fr deflectable guiding catheter, which is the key step of the procedure, and assessment of the correct annular puncture site at the posterior annulus (P1 and P3 scallop regions). The annular puncture and plication process can be monitored using live 3D mode which permits real-time visualisation of annuloplasty and final assessment of plication results. Colour Doppler is used for the evaluation of residual MR. Anterior-posterior and septal-lateral dimensions for the determination of induced mitral valve geometry changes can be obtained from intercommissural and 4-chamber/long-axis views, respectively.
Safety and functional outcomes after a Mitralign procedure
Twenty-one of the patients included in the first-in-man trial could not receive any implants. If completed with the implantation of two pairs of pledgets, the Mitralign procedure induces reverse LV remodelling, in line with the reduction of MR (Figure 1). It seems reasonable to assume that the study results would have been more favourable if every patient included had received device treatment with multiple pairs of pledgets. Of note, there were two deaths within 30 days in patients with an ejection fraction below 30% in the first-in-man trial. In patients in whom two pairs of pledgets could be successfully deployed, a pronounced reduction of MR and MV annular dimensions was observed.
Recently, six-month safety and clinical outcome results were reported in 71 patients with moderate to severe FMR undergoing annuloplasty with the Mitralign system. All-cause mortality, stroke and myocardial infarction rates were favourable six months after the procedure (12.2%, 4.9% and 0%, respectively), while echocardiographic core lab analysis showed significant MR reduction in 50% of treated patients.
The Mitralign procedure significantly reduced anterior-posterior (–0.31±0.4 cm, p<0.01) and septal-lateral dimensions (–0.21±0.3 cm, p<0.01), and increased MV coaptation length (0.13±0.2 cm, p<0.01). The treatment induced reverse LV remodelling with reduction of LV end-diastolic diameter (–0.20±0.4 mm, p=0.03), and volume (–22±39 ml, p=0.01), and was furthermore associated with significant improvement in six-minute walking distances (56.5±92.0 metres, p=0.01) and functional NYHA class.
In conclusion, percutaneous annuloplasty with the Mitralign system is feasible and safe in high-risk FMR patients. Pre-procedural imaging and clinical evaluation are crucial for determination of patient eligibility and assessment of mitral and ventricular geometry and dimensions.
CT and echocardiography aid in determining patient suitability and planning for annular puncture location and device deployment.
Further careful refinement of patient selection seems an important issue for future studies and clinical application of the Mitralign device.
Conflict of interest statement
The authors have no conflicts of interest to declare.

